Abstract
Over the past few decades, lifespans of sickle cell disease (SCD) patients have increased; hence, they encounter multiple complications. Early detection, appropriate comprehensive care, and treatment may prevent or delay onset of complications. There is a gap in the literature describing the SCD complication rates, blood transfusion patterns, iron chelation therapy (ICT) use, and associated resource utilization in SCD patients ≥16 years old. This study contributes to addressing this gap.
Medical records of 254 SCD patients ≥ 16 were retrospectively reviewed between August 2011 and July 2012 at three US tertiary care centers (University of Tennessee: 117; Tulane University: 72; Howard University: 65). Data were collected from patient's first visit after age 16 (index date) until the earliest indication of death, loss to follow-up, or last patient record on file prior to the centers' IRB submission dates. Patients were classified into one of three cohorts based on cumulative units of blood transfused and history of ICT: <15 units of blood and no ICT (minimally transfused, Cohort 1 [C1]), ≥15 units of blood and no ICT (Cohort 2 [C2]), and ≥15 units of blood and receiving ICT (Cohort 3 [C3]). SCD complication rates were expressed as the number of SCD complications recorded from patient charts per patient per year (PPPY) and compared among cohorts using rate ratios (RRs).
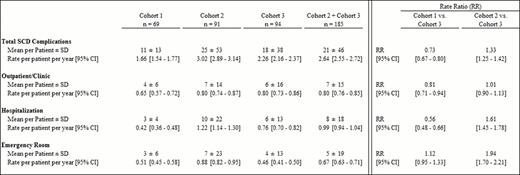
Cohorts 1, 2, and 3 consisted of 69, 91, and 94 patients, respectively. Mean (range) age at index date was similar across cohorts (27 yrs [16–65]) and all patients were African American. Mean length of observation was shorter among patients in C1 (yrs, C1: 6.6; C2: 8.2; C3: 8.1). Post index date, patients in C1 received an average of 1 unit of blood PPPY (p<0.001 vs. C2 and C3), whereas patients in C2 and C3 received an average of 10 and 15 units PPPY (p=0.112), respectively. Among patients with serum ferritin (SF) assessment within 60 days before ICT (n=57), mean (median) SF level was 4,881 ng/mL (4,040). Across all three cohorts, the most common SCD complication was acute pain crisis (69.8%), followed by infection/sepsis (5.1%), leg ulcers (2.9%), and avascular necrosis (2.3%). The rate (95% CI) of any SCD complications was the highest in C2 at 3.02 PPPY (2.89–3.14), followed by 2.26 PPPY (2.16–2.37) in C3, and 1.66 PPPY (1.54–1.77) in C1 (Table 1). Among transfused patients (C2+C3), those receiving ICT were less likely to experience SCD complications than those who did not (RR [95% CI] C2 vs. C3: 1.33 [1.25–1.42]). Similar trends (RR [95% CI]) were observed in emergency room (ER) visits and hospitalizations associated with SCD complications (C2 vs. C3, ER: 1.94 [1.70–2.21]; hospitalizations: 1.61 [1.45–1.78]), but not in outpatient visits.
Results from this study highlight the significant burden of complications and the associated healthcare resource utilization for SCD patients. The results suggest that among regularly transfused patients, those who received ICT were less likely to experience complications than those without ICT. However, transfusions are not necessary for all patients with SCD and patients with more complications may have started transfusion therapy earlier. Patients receiving ICT may also receive closer monitoring, which may help with early identification and intervention to delay or prevent the development of complications and improve outcomes.
Jordan:Novartis Pharmaceuticals Corporation: Consultancy, Speakers Bureau. Oneal:Novartis Pharmaceuticals Corporation: Honoraria. Vekeman:Novartis Pharmaceuticals: Research Funding. Bieri:Novartis Pharmaceuticals Corporation: Research Funding. Sasane:Novartis Pharmaceuticals: Employment. Marcellari:Novartis Pharmaceuticals Corporation: Employment. Magestro:Novartis Pharmaceuticals: Employment. Gorn:Novartis Pharmaceuticals Corporation: Research Funding. Duh:Novartis Pharmaceuticals: Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.