Abstract
Abstract 2319
Granulocyte-colony stimulating factor (G-CSF) is the prototypic agent used to mobilize hematopoietic stem and progenitor cells (HSPCs) into the blood where they can then be harvested for stem cell transplantation. G-CSF acts in a non-cell-intrinsic fashion to induce HSPC mobilization. We recently showed that G-CSF signaling in a CD68+ monocyte/macrophage lineage cell within the bone marrow initiates the HSPC mobilization cascade (Christopher et al., 2011). Consistent with this finding, two other groups showed that ablation of monocytes/macrophages induces HSPC mobilization (Winkler et al., 2010; Chow et al., 2011). CD68 marks a heterogeneous cell population that includes monocytes, macrophages, myeloid dendritic cells, and osteoclasts. To further define the relevant cell population(s) for HSPC mobilization by G-CSF, we first examined the role of osteoclasts. Receptor activator of NF-kappaB (RANK) signaling is required for osteoclast development. Osteoprotegerin (OPG) is a decoy receptor for RANK ligand, and treatment with OPG-Fc (a stabilized form of OPG) results in osteoclast ablation in mice. We treated mice with 100 μg of OPG-Fc and documented complete osteoclast ablation by histomorphometry. Osteoclast ablation did not result in constitutive HSPC mobilization, nor did it affect G-CSF-induced HSPC mobilization. To further assess the role of osteoclasts, we transplanted RANK−/− fetal liver cells into irradiated Csf3r−/− (G-CSF receptor deficient) recipients. Since RANK is required for osteoclast development, the osteoclasts in these bone marrow chimeras lack the G-CSFR, while other hematopoietic cells (including monocytes/macrophages) are G-CSFR sufficient. Again, G-CSF-induced HSPC mobilization in these mice was normal. Based on these data, we conclude that osteoclasts are dispensable for HSPC mobilization by G-CSF.
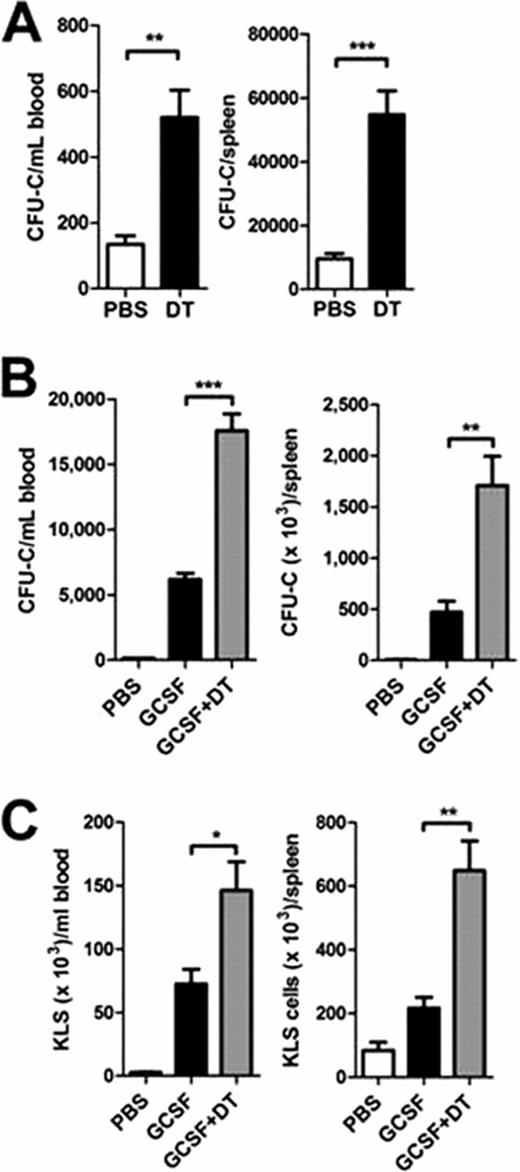
We next quantified changes in monocytic/macrophage cell populations in the bone marrow after G-CSF treatment (250 μg/kg per day for 5 days) using a novel multi-color flow cytometry assay that includes CD115, F4/80, MHC class II, Gr-1, B220, and CD11c. Using this assay, we observed a significant decrease in macrophages (11.8 ± 3.6-fold) and, surprisingly, myeloid dendritic cells (MDCs; 5.5 ± 1.2-fold) in the bone marrow with G-CSF treatment. To further assess the role of MDCs, we used transgenic mice expressing the diphtheria toxin receptor under the control of the CD11c promoter (CD11c-DTR) to conditionally ablate MDCs. To avoid systemic toxicity, we transplanted CD11c-DTR bone marrow into congenic wild type recipients prior to MDC ablation. The resulting bone marrow chimeras were treated with diphtheria toxin (DT; 400 ng per day for 6 days), which resulted in a 92% reduction in MDCs. Ablation of MDCs resulted in a significant increase in colony-forming cells in the blood and spleen (figure 1A). Moreover, MDC ablation significantly increased mobilization of colony-forming cells and c-Kit+lineage−Sca-1+ (KLS) cells by G-CSF (figures 1B and 1C). Taken together, these data suggest that myeloid dendritic cells, but not osteoclasts, contribute to HSPC mobilization by G-CSF.
HSPC mobilization in CD11c-DTR mice. CD11c-DTR bone marrow chimeras were treated with diphtheria toxin (DT) alone, G-CSF alone, or DT plus G-CSF. The number of CFU-C (A & B) or KLS cells (C) in the blood and spleen are shown. Data represent the mean ± SEM of 10–11 mice pooled from two independent experiments. *p < 0.05; **p < 0.001; ***p < 0.0001.
HSPC mobilization in CD11c-DTR mice. CD11c-DTR bone marrow chimeras were treated with diphtheria toxin (DT) alone, G-CSF alone, or DT plus G-CSF. The number of CFU-C (A & B) or KLS cells (C) in the blood and spleen are shown. Data represent the mean ± SEM of 10–11 mice pooled from two independent experiments. *p < 0.05; **p < 0.001; ***p < 0.0001.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.