Abstract
Abstract 2346
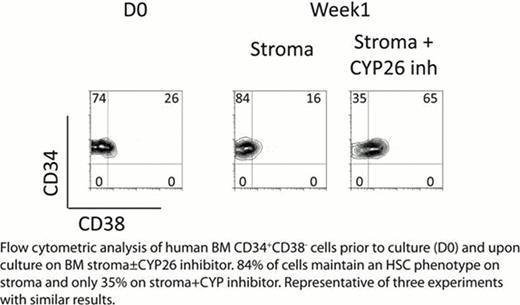
HSCs reside in a complex microenvironment that assures their survival and is presumably essential for maintaining the physiologic balance between self-renewal and differentiation. Disruption of HSC-niche interactions, as occurs during ex vivo culture, generally results in rapid loss of HSC activity, although non-physiologic conditions have been reported to support ex vivo expansion of mouse or cord blood HSCs. However, there has been little success expanding BM-derived human HSCs ex vivo. Thus, the physiological mechanisms responsible for human HSC self-renewal remain ambiguous. RA levels, under the control of the fetal gonad niche, have been shown to control germ cell fate decisions; specifically, Sertoli cells act as a sink for RA through their expression of the cytochrome P450 enzymes CYP26 which inactivate retinoids (Bowles et al Science 2006). We investigated whether microenvironmental control of RA activity might also play a role in physiologic HSC fate decisions. Culturing human phenotypic HSCs (CD34+CD38−) from BM with the pan-RA receptor antagonist AGN194310 (AGN) resulted in delayed generation of total cell numbers and colony forming unit cells (CFU-C) during day (D) 7–14 of culture, but ultimately statistically higher overall cellular and CFU-C output at D28 when compared to control (serum-free, thrombopoietin, kit ligand, and Flt3 ligand or TSF) (p=0.04). AGN also significantly expanded CD34+CD38− cells as well as the primitive week 8 cobblestone area forming cells (CAFCW8) compared to control (D7 CAFCW8 expansion in TSF+AGN vs TSF - 12±5 vs 8±3 fold change from D0, D14 – 10±3 vs 6±2, and D21 – 2.4±2 vs 0.7±0.3, p<0.05 at all time points). Most importantly, AGN treatment of human BM CD34+CD38− cells enhanced their engraftment in NOD/SCID/IL2Rg−/− mice, with significant expansion of SCID-repopulating cells (SRC): TSF+AGN vs TSF D7 SRC expansion - 4.1±1.3 vs 1.4±0.3 fold change from D0 and D14 – 1.6±0.2 vs 0.4±0.1 (p<0.05 at both time points for 3 independent experiments at 4 months after transplant). As the best conditions to maintain human BM HSCs in vitro remain stromal based cultures, we studied if BM stroma may provide a low RA sanctuary for HSCs. BM stromal cell lines and fresh explanted adherent stroma layers were found to express high levels of the retinoid-inactivating enzyme CYP26. Using a RA responsive Luciferase reporter, conditioned media from BM stroma showed significantly (p=0.01) lower levels of RA activity than fresh media (with 10% serum which contains mM levels of vitamin A and nM levels of RA); this decrease in RA activity was significantly reversed by incubation of BM stroma with the specific CYP26 inhibitor R115866 (Rambazole) (p<0.01) or CYP26 knockdown with siRNA. The rapid loss of HSC phenotype during serum-containing culture of BM CD34+CD38− cells was significantly delayed by AGN or co-culture with BM stroma. Incubation of stroma with the specific CYP26 inhibitor reversed the ability of stroma to maintain the HSC phenotype (Figure) and function (D21 stroma vs stroma + CYP inhibitor CAFCW8 numbers - 0.72±0.1 vs 0.52±0.04 fold change from D0, p=0.03). These data suggest that HSCs, unless specifically prevented from doing so, are intrinsically programmed to undergo differentiation, at least in part by their high expression of aldehyde dehydrogenase 1 (i.e., retinaldehyde dehydrogenase), the rate limiting step in RA biosynthesis. The BM microenvironment, via expression of CYP26, protects HSCs from the pro-differentiation effects of retinoids, thus promoting their self-renewal. These results may also suggest a more general mechanism by which the BM microenvironment, via expression of cytochrome P450 enzymes, protects normal and malignant HSCs from environmental stressors, including cytotoxic agents.
No relevant conflicts of interest to declare.