Abstract
Abstract 2609
Children with relapsed or chemotherapy-refractory ALL have a poor prognosis despite the use of aggressive therapies such as HSCT. Chimeric antigen receptor modified T cells targeting the B-cell antigen CD19 have been reported to be effective in adults with B-cell lymphomas and chronic lymphocytic leukemia. We conducted pre-clinical studies with a CD19-CAR consisting of a CD19-specific scFv and the CD28 and CD3z signaling domains. These cells generated significant levels of IFNg, TNFa, and IL-2 in response to ALL blasts and rapidly eradicated human ALL in murine xenografts. We developed a Phase I clinical trial of CD19-CAR modified autologous T cells for children with CD19+ hematologic malignancies. HSCT-na•ve and post-transplant patients are eligible, and cells are collected directly from patients in both cases. CD19-CAR T cells are manufactured in a semi-closed system over an 11-day period. We report results with the first patient, a 13-year old with chemotherapy-refractory ALL that had relapsed after 2 prior matched related donor HSCTs.
Peripheral blood (PB) mononuclear cells were collected from the patient on Day -11 by apheresis. T cells were positively selected and activated by incubation with anti-CD3/anti-CD28 paramagnetic beads in IL-2 for 48 hours then transduced with the CD19-CAR gene via retroviral supernatant for an additional 48 hours. Beads were removed and the expanding CD19-CAR T cells were maintained in culture with IL-2 until harvested for infusion on Day 0. A 59-fold expansion of CAR T cells with 65% transduction efficiency was achieved. The patient was pre-treated with fludarabine (25 mg/m2/day on Days -4, -3, -2) and cyclophosphamide (900 mg/m2 on Day -2) prior to the infusion of 1×106 CAR-transduced T cells/kg.
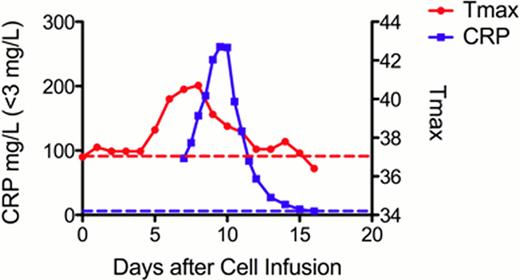
The patient developed signs and symptoms of cytokine release syndrome (CRS) on Day +5 with full resolution by Day +11. Manifestations included fever (maximum 41°C), rigors (Grade 1), and hypotension (Grade 2), the latter of which was responsive to two IV fluid boluses. The patient also developed an erythematous rash (Grade 1) of the extremities from Days +7 to +12 and bilateral scrotal swelling and pain (Grade 1) from Days +8 to +10, which was associated with increased testicular blood flow by ultrasound. Cytokine analysis revealed high levels of IL-6 (53.1 pg/ml; normal <5), GM-CSF (59.4 pg/ml; normal <7.8) and IFNg (44.7 pg/ml; normal <15.6). As IL-6 induces release of C-reactive protein (CRP) from the liver, we monitored CRP levels, which mirrored the time course of CRS (Figure ). Broad-spectrum antibiotics were administered for neutropenic fever, although all cultures were negative. No other therapeutic interventions were provided aside from routine supportive care.
Maximum temperature in each 24-hour period (Tmax, red solid line) and CRP (blue solid line) correlate with cytokine release syndrome. Dotted lines indicate upper limits of normal. PB flow cytometry performed prior to cell infusion demonstrated 0.04% blasts and 1% normal B-cells. Repeat analysis after infusion revealed clearance of blasts, gradually decreasing B cells, and a maximum of 0.07% CD19CAR T cells (Days +3, +6, and +27). Re-staging evaluation on Day +27 revealed achievement of a complete remission with bone marrow (BM) blasts decreasing from 30% pre to 3% (flow cytometry 5% to 0.6%) and cerebrospinal fluid (CSF) blasts decreasing from 1.5% to 0%. 0.4% of BM T cells expressed the CD19CAR. (Table)
Maximum temperature in each 24-hour period (Tmax, red solid line) and CRP (blue solid line) correlate with cytokine release syndrome. Dotted lines indicate upper limits of normal. PB flow cytometry performed prior to cell infusion demonstrated 0.04% blasts and 1% normal B-cells. Repeat analysis after infusion revealed clearance of blasts, gradually decreasing B cells, and a maximum of 0.07% CD19CAR T cells (Days +3, +6, and +27). Re-staging evaluation on Day +27 revealed achievement of a complete remission with bone marrow (BM) blasts decreasing from 30% pre to 3% (flow cytometry 5% to 0.6%) and cerebrospinal fluid (CSF) blasts decreasing from 1.5% to 0%. 0.4% of BM T cells expressed the CD19CAR. (Table)
| Day . | % BM Blasts (Flow) . | BM CAR % of T cells . | % PB Blasts . | PB CAR % of T cells . | PB B Cells % . | % CSF Blasts . | % CSF CAR . |
|---|---|---|---|---|---|---|---|
| Pre | 30 (5) | 0 | 0.04 | 0 | 1 | 1.5 | 0 |
| +3 | 0 | 0.07 | 0.9 | ||||
| +6 | 0 | 0.07 | 0.2 | ||||
| +13 | 0 | 0 | 0.1 | ||||
| +27 | 3 (0.6) | 0.4 | 0.01 | 0.07 | 0.06 | 0 | 0 |
| Day . | % BM Blasts (Flow) . | BM CAR % of T cells . | % PB Blasts . | PB CAR % of T cells . | PB B Cells % . | % CSF Blasts . | % CSF CAR . |
|---|---|---|---|---|---|---|---|
| Pre | 30 (5) | 0 | 0.04 | 0 | 1 | 1.5 | 0 |
| +3 | 0 | 0.07 | 0.9 | ||||
| +6 | 0 | 0.07 | 0.2 | ||||
| +13 | 0 | 0 | 0.1 | ||||
| +27 | 3 (0.6) | 0.4 | 0.01 | 0.07 | 0.06 | 0 | 0 |
A complete remission was successfully induced in a child with chemotherapy-refractory ALL and post-transplant relapse using a single infusion of autologous-collected, donor-derived T cells modified with a CD19-CAR. Treatment was well tolerated and was only associated with mild CRS that was characterized by high levels of IL-6, INFg, and GM-CSF but not IL-1b or TNFa. CRP levels can be used as a readily available biomarker of IL-6-associated CRS. To our knowledge, this is the first CAR-based therapy to be utilized in the post-allogeneic setting using donor-derived T cells collected directly from a pediatric patient.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.