Abstract
Abstract 2635
EBV+ Hodgkin lymphoma (HL) arises from a latently EBV infected B cell and is associated with infectious mononucleosis (IM), a febrile illness that presents upon primary EBV infection of B cells in a proportion of adolescents and young adults. After primary infection, the virus establishes lifelong persistence in a small number of circulating B cells and this number is strongly controlled by the immune system, with a prominent role for HLA class I restricted recognition of EBV antigenic peptides by cytotoxic CD8 T cells.
Interestingly, in the western European population, HLA-A1 is a susceptibility type for EBV+ HL, while HLA-A2 is protective. This corresponds well to known HLA restricted immune responses to EBV in healthy individuals, with consistent cytotoxic responses in the context of HLA-A2 and no responses in the context of HLA-A1. Because EBV+ HL tumor cells usually express all components of the antigen presenting pathway, including HLA class I, the effect of HLA type on susceptibility may act at any stage of disease pathogenesis. We therefore tested 2 hypotheses in this study: 1. HLA-A type is associated with IM risk and 2. HLA-A type determines EBV+ HL risk by influencing the number of circulating EBV+ B cells.
To test the first hypothesis a total of 158 consecutive IM patients diagnosed from 1993 to 2011 at the Queen Elizabeth Hospital Birmingham, United Kingdom were included. These were all Caucasians patients with a clinical diagnosis of IM, including positive EBV antibody titers and/or Monospot test. HLA class I typing (HLA-A, B and C) was performed and allele frequencies were compared to 12,762 regional Blood Bank volunteers. The HLA-A1 type was clearly under represented in IM patients (12.3%) compared to controls (19.3%, OR 0.59; 95%CI 0.42–0.83), in sharp contrast to the situation in EBV+ HL. The uncommon HLA-B53 type was overrepresented in IM patients (1.6% vs. 0.3%, OR 5.313; 95%CI 2.14–13.2). Some other types showed borderline significance: HLA-A3 (OR 1.41; 95%CI 1.07–1.87), HLA-B14 (OR 1.68, 95%CI 1.07–2.62), HLA-C7 (OR 0.75; 95%CI 0.58–0.97) and HLA-C8 (OR 1.76, 95%CI 1.10–2.81). These data indicate that the known association of HLA-A1 and HLA-A2 with EBV+ HL is not paralleled by a similar association with IM. The unexpected negative correlation between HLA-A1 and IM may be caused by an association with less severe IM symptoms as suggested in another study (McAulay et al J Clin Invest 2007), if individuals with less severe IM symptoms did not seek medical attention.
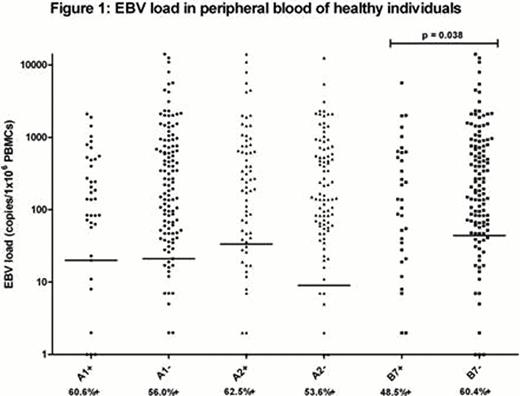
To answer the second hypothesis, first year medical students from the University of Birmingham, UK were asked to participate and donate blood in the years 2007 and 2008. EBV viral load was measured by Q-PCR using PBMC DNA from 270 students with positive EBV serology (30% of all students were serology negative) and no recent IM symptoms. Viral load was considered indicative for number of circulating EBV+ B cells. HLA-typing was done for HLA-A1, A2, B7, B8 and B35. For each type EBV loads were compared between HLA type carriers and non-carriers. Results with medians are shown in figure 1. The percentages of EBV seropositive students with detectable EBV loads per HLA-type are indicated under the x-axis (57.5% for all subjects). EBV seropositive students with undetectable EBV loads were also included in the statistical analyses. Of the individuals with HLA types previously shown to be involved in cytotoxic immune responses to latent EBV (A2, B7, B8 and B35), only HLA-B7 positive individuals had a significantly lower viral load than HLA-B7 negative individuals (p=0.038, median: 0 vs. 44). None of the other HLA types showed a significant difference between HLA type carriers and non-carriers, including HLA-A1 (expected to have higher viral loads in A1+ donors).
In conclusion, the risk effect of HLA-A1 and the protective effect of HLA-A2 in the development of EBV+ HL are unlikely to be explained by differences occurring during primary EBV infection or differences in the lifelong virus-host balance. Instead, these effects probably relate to immune surveillance of EBV infected Hodgkin (precursor) cells at later stages of disease pathogenesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.