Abstract
Abstract 269
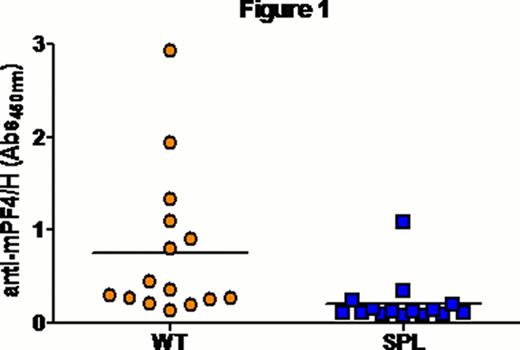
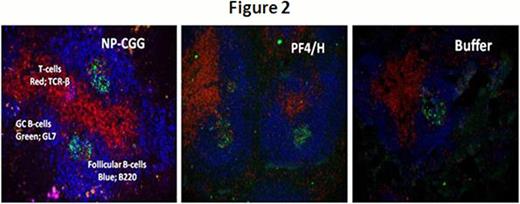
Mice injected with ultra-large complexes (ULCs) of platelet factor-4 (PF4) and heparin (PF4/H) exhibit a strong immune response which mimics that seen in patients developing Heparin induced thrombocytopenia (HIT)(Suvarna, Blood 2005). Previous studies with this murine immunization model have shown that murine (m) PF4/H ULCs potently activate dendritic cells (DCs) (Chudasama, Blood 2010) and initiate a T-cell dependent immune response (Suvarna, Blood 2005). To examine cellular/anatomic basis of PF4/H antibody formation, we examined the role of the spleen and germinal center formation in mice injected with mPF4/H complexes. To evaluate the role of the spleen, we immunized splenectomized mice (SPL) (n=15) and wild type (WT) mice (n=15) retro-orbitally with 100 mg/mL mPF4 and 5 U/mL Hep for 5 days. Antibodies to mPF4/H complexes were detected by an ELISA developed in our laboratory (Suvarna, Blood 2005; Suvarana, Blood 2007). As shown in Figure 1, SPL animals showed a marked reduction in Ab formation compared to WT animals (mean A450nm ± SD; SPL 0.206 ± 0.253 vs. WT 0.76 ± 0.788, p<0.003). We next asked if antibody responses to PF4/H were associated with germinal center (GC) formation. The GC is a hallmark of T-cell dependent (TD) immune responses and is considered vital for development of high-affinity and isotype-switched antibodies. For these studies, mice were injected with a high dose of PF4/H (400 ug/mL PF4: 10 U/mL heparin) (n=2), standard protocol dose of PF4/H (100 ug/mL: 5 U/mL) and or buffer (Hank's Balanced Salt Solution, n=3) daily for 5 days via retro-orbital injection. As a positive control for GC formation, we injected two mice with a conventional antigen, nitrophenyl conjugated chicken gamma-globulin (NP-CGG). Mice expressing high antibody titers on Day 16 (mean A450nm± SD; high dose- 1.112 ± 0.5767; standard dose- 1.186 ± 0.7853) were sacrificed, spleens harvested and frozen in an embedding medium. Splenic sections (5m, longitudinal) were obtained as frozen sections and stained with immunohistochemical markers for GCs (GL-7 FITC; 1:400, counter-stain- FITC Alexa Fluor 488; 1:200), T-cells (TCR-β PE; 1:200) and B-cell follicles (B220 Biotin; 1:400, counter-stain- Streptavidin Alexa Fluor 350; 1:200). Images were obtained using Carl Zeiss AxioVert 200M microscope and analyzed with AxioVision Rel 4.6 software. Fluorescent images were processed with Adobe photoshop CS5.1. As shown in Figure 2, we noted that mice injected with NP-CGG, had ∼ 22 GC's, mice injected with buffer had 2 GC's and mice injected with high and standard dose of PF4/H, had ∼20–22 and 15–17 GC's respectively. These studies show that mPF4/H complexes elicit GC formation in association with secretion of mPF4/H Abs and that GC formation is comparable to that seen with conventional antigen.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.