Abstract
Abstract 2694
A number of tumor molecular markers have been shown to predict the clinical course and overall survival (OS) in non-Hodgkin lymphoma (NHL). Additionally, several lifestyle factors (e.g., obesity, alcohol use, and diet) have also been shown to affect the outcome of patients with NHL. However, potential interactions and added prognostication connecting lifestyle factors and tumor markers have not been studied.
To assess the association between lifestyle factors (prior to NHL diagnosis) and pathologic tumor markers (i.e., tumor-host microenvironment and cellular and molecular markers) and to examine the impact of these markers on survival in FL, we conducted a population-based prospective study on 123 FL patients diagnosed between 1999 and 2002; patients were followed through 2011. We performed immunohistochemical staining on tissue microarrays (TMAs) from lymph nodes to evaluate prognostic tumor markers for FL (CD68, CD7, FOXP3, CD10, and Ki67); scoring was performed by expert hematopathologists (AC, DDW). Lifestyle habits (i.e. smoking, diet, alcohol use, and smoking) prior to NHL diagnosis were assessed through comprehensive validated questionnaires (e.g., Health Habits and History Questionnaire). Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated utilizing multivariate Cox regression.
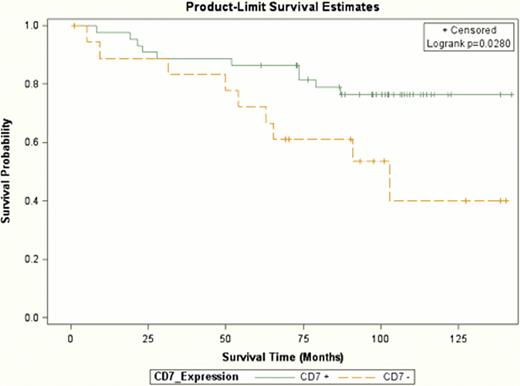
The median age of patients was 60 years (48–70), 89% had advanced-stage disease, and 49% of patients were female. The mean patient follow-up was 8.1 years. High microenvironment expression of CD7 was significantly associated with a dietary pattern high in fruits, vegetables, and starch (p=0.04), as well as more specifically a higher intake of carotene-rich vegetables (p=0.005). Additionally, ‘never smokers’ more frequently had increased microenvironment expression of CD7 compared with ever smokers (89% vs 58%, respectively, p=0.02). Furthermore, survival analyses showed that FL patients with high CD7+ expression had a significantly better OS (HR=0.34 [95% CIs 0.14–0.84], P=0.02, for all-cause mortality) compared to those patients with low CD7 expression (see Figure). The association was not attenuated after adjustment for intake of fruits or vegetables overall or carotene-rich vegetables. However, the impact of the HR for CD7+ expression was decreased after adjustment for smoking status (HR=0.4; CI=0.1–1.30). We also found that FL patients who were above the median intake for fruit were more likely to have CD10+ tumor cell expression (OR=5.3 [95% CIs 1.0–26.5], P=0.04) compared with those below the median intake. The other FL tumor markers examined (i.e., CD68, FOXP3, and Ki67) had no impact on OS nor did they associate with the other lifestyle factors evaluated.
To our knowledge, this represents the first report of the association of lifestyle factors on tumor markers/microenvironment in NHL and their collective impact on survival. We identified several novel lifestyle/tumor marker associations including the association of CD7+ microenvironment expression with diets high in fruits and vegetables and with never smokers. CD7+ T-cells are a component of the favorable FL immune response-1 signature (Dave S, NEJM, 2004); it is possible that smoking and/or dietary intake skew the host T-cell lymphocyte milieu towards an adverse immune imbalance. Furthermore, we found that CD7 expression was associated with improved OS independent of age and sex; however, its predictive value was slightly attenuated after adjustment for smoking. Continued examination of the interactions of lifestyle factors with tumor markers and the related impact on patient survival are warranted.
Overall survival of FL patients based on expression of CD7 tumor-related microenvironment cells.
Overall survival of FL patients based on expression of CD7 tumor-related microenvironment cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.