Abstract
Abstract 2858
Janus-activated kinase 2 (JAK2) gene mutations and translocations are involved in the pathogenesis of a variety of hematologic malignancies. Among different translocation partners, pericentriolar material 1 (PCM1)-JAK2 fusion products have been described in rare cases of both lymphoid and myeloid neoplasms characterized by morphological (myeloproliferaton, eosinophilia, myelofibrosis) and clinical (striking male predominance, aggressive course) similarities.
We recently identified a new case of the rare translocation PCM1-JAK2 in a 29-year-old man presenting with atypical chronic myeloid leukemia (aCML) and peculiar aspects of diserythropoiesis in the bone marrow (BM): abundant paratrabecular clusters of proerythroblasts associated with marked reduction of mature erythroid compartment (Sammarelli et al., S.I.E.S. 12th Meeting, 2012).
For the first time we describe here the erythroid differentiation capacity of ex-vivo expanded CD34+ cells from this PCM1-JAK2 fusion case, as well as the signaling pathways activated in peripheral blood neoplastic cells (PBNC) harboring the translocation.
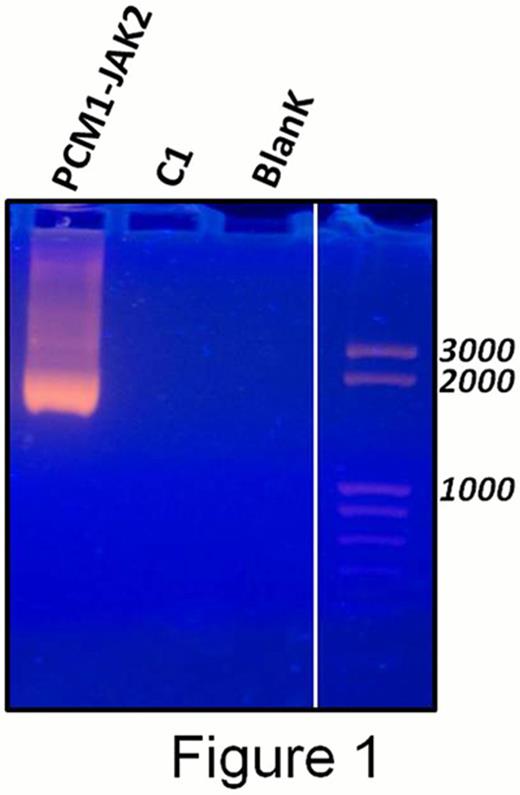
Presence of the PCM1-JAK2 fusion transcript in PBNC was confirmed by nested RT-PCR using primers derived from PCM1 exon 25 and JAK2 exon 9 described in Reiter et al. Cancer Res. 2005 (Figure 1).
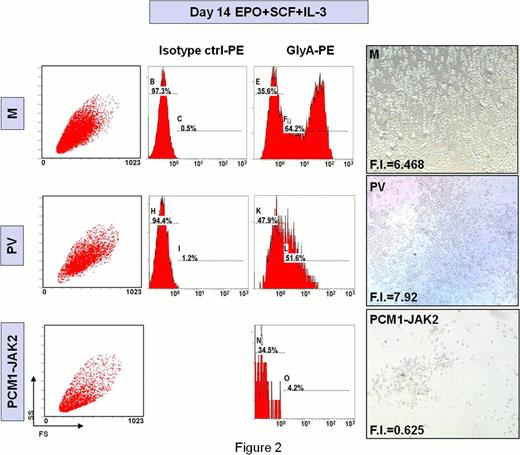
CD34+ cells were isolated from the peripheral blood (PB) of the patient and cultured in serum-free medium supplemented with erythropoietin (EPO), interleukin-3 (IL-3) and stem cell factor (SCF) to induce erythroid differentiation; erythroid cell output [evaluated in terms of fold increase (FI) and glycophorin-A (GlyA) expression at day 14 of culture] was compared to the one obtained from PB CD34+ cells from a polycythemia vera patient (PV), in which JAK2 is constitutively activated by V617F point mutation, and to CD34+ cells from a G-CSF-mobilized donor (M).
As shown in Figure 2, FI and GlyA expression were significantly lower in our patient compared to M and PV (FI: 0.63, 6.47 and 7.92 respectively; GlyApos cells: 4.2%, 51.6% and 64.2%, respectively) consistently with the diserythropoietic picture in the BM.
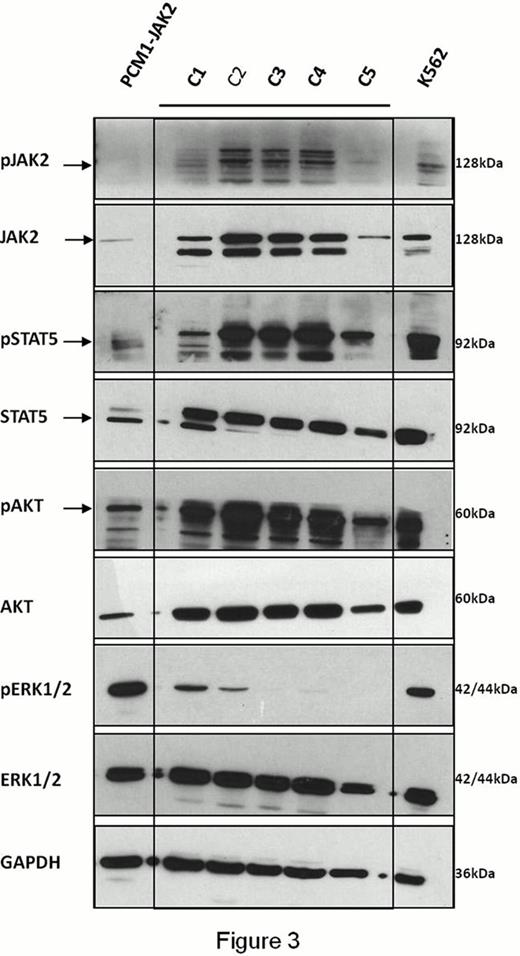
We then investigated the activation of the 3 main signaling pathways associated to Receptor tyrosine kinases and most commonly turned on in cancer: Mitogen-activated protein (MAP) kinase pathway, JAK/Signal transducer and activator of transcription (STAT) pathway and phosphatidylinositol 3-kinase (PI3K)/AKT pathway, evaluating, by Western Blot analysis, levels of phosphorylation of Extracellular signal-Regulated Kinase (ERK1/2), JAK2, STAT5 and AKT in PBNC from our PCM1-JAK2 case and in PB mononuclear cells (PBMC) from 5 healthy control subjects (C1-C5).
Although these signaling cascades are deeply interconnected, we surprisingly found a selective activation of the sole MAP-kinase pathway in PBNC (Figure 3).
These data suggest that, while presence of JAK2V617F mutation leads to ligand-independent activation of STAT5, AKT and ERK1/2 (Laubach et al. Exp. Hematol. 2009), PCM1-JAK2 fusion product fails to activate JAK/STAT and PI3K/AKT axis. Specifically, reduced STAT5 activation might explain impaired erythroid differentiation of CD34+ cells in vitro as well as the marked aspects of diserythropoiesis in the BM.
The signaling signature of PMC1-JAK2 neoplastic cells described here has also relevant implications on the treatment strategy for these patients. In particular, given the lack of activation of JAK2 and its down-stream partner STAT5, JAK-inhibitor therapy does not seem the ideal candidate in this specific setting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.