Abstract
Abstract 3283
Donor-derived regulatory T cells (Treg) and natural killer (NK) cells can respectively improve stem cell transplant (SCT) outcome by reducing graft versus host disease (GVHD) severity and exerting a graft-versus-leukemia effect. High frequencies of donor Treg are associated with less GVHD, and low doses of interleukin-2 (IL-2) can expand both NK and Treg after allogeneic SCT. To explore the feasibility of improving the quality of peripheral blood SCT donations, we evaluated the safety and the tolerability of ultra-low dose IL-2 administration to volunteers with the aim of preferentially expanding Treg and NK cells.
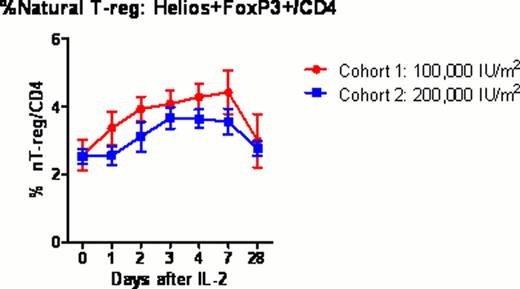
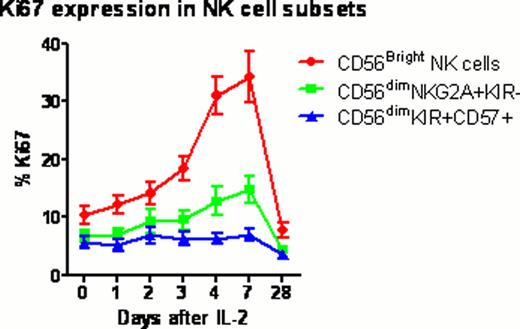
Twelve healthy volunteers (mean age 34 years; range 22–57) received 0.1 or 0.2 million U/m2/day IL-2 subcutaneously for 5 days (NIH protocol 11-H-0268). Blood samples were collected before and 1, 2, 3, 4, 7 and 28 days after IL-2 injection. Samples were analyzed by multiplex techniques including whole transcriptome gene expression with HumanGene 1.0ST microarrays; serum levels of 69 cytokines and chemokines by Luminex assay; and lymphocyte phenotyping by flow cytometry, to comprehensively characterize the cellular and molecular immune response to IL-2 (“IL-2 immunome”). Treg subsets were determined within the CD4+ T cell population using FoxP3, Helios, CD45RA and CD31 to identify thymus-derived natural Treg (nTreg), induced Tregs (iTreg) and their recent thymic emigrants (RTE). NK cell subsets were determined within CD56+CD3- population using NKG2A, KIR2DL1, KIR2DL2/3, KIR3DL1 and CD57 to identify CD56bright, CD56dim NKG2A+KIR-, and CD56dim KIR+CD57+ cells.
All subjects tolerated ultra-low dose IL-2 with minimal adverse events (mainly grade 1–2 injection site reactions). The fraction of FoxP3+Treg in CD4 rose significantly above baseline peaking at 4 days (3.7% vs 5.8%; p=0.0004) after the first dose of IL-2. Treg subset analysis demonstrated that the fraction of nTreg and RTE nTreg in CD4 expanded significantly in the lower dose cohort compared to the higher dose cohort (p=0.004 and p=0.005 respectively). %CD56bright NK significantly increased at 7 days (p=0.008), whereas CD56dimNKG2A+KIR-, and CD56dimKIR+CD57+ NK cells remained at baseline. The Ki67 proliferation marker further verified a significant in vivo expansion of CD56bright NK cells with ultra-low dose IL-2. Cytokine and chemokine profiling demonstrated significant increase circulating level of IP-10 (P=0.0018) through day 2 to 4 after IL-2 injections. In contrast, circulating levels of IL-2, IL-6, IL-10, IL-15 and IL-17 remained unchanged after IL-2 injection. Gene expression microarray studies revealed significant changes in 24 genes (P value < 0.1 corrected by false discovery rate (FDR) for multiple testing), including up-regulation of IL-2RA and FOXP3 as early as 2 days after IL-2 injections. Gene Set Analysis (GSA) revealed significant changes (P value < 0.1 after FDR) in innate immune response pathways, including Toll-like receptor signaling and interferon signaling.
This is the first study to show that ultra-low dose IL-2 could be safely administrated to healthy volunteers to expand thymic-derived natural Treg and CD56bright NK cells. These results raise the possibility of using ultra-low dose IL-2 to boost Treg and NK cells in stem cell donors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.