Abstract
Abstract 3378
Nonacog alfa (Benefix®) has been available in Japan since January 2010 and is being used widely for prophylaxis in patients with hemophilia B as the first recombinant factor IX. We have been conducting a surveillance study in order to investigate adverse events, safety and efficacy with routine use conditions in all patients receiving Benefix. The recovery of recombinant factor IX was measured to assess factors affecting appropriate dosing of nonacog alfa.
All patients who were administered Benefix were enrolled in this study by central registration system until 300 patients were registered. The initial dose was around 50 IU/kg, but subsequent doses may have been modified according to condition of the patient and recovery. Observation period was 12 and 24 months for previously treated patient (PTP) and previously untreated patients (PUP), respectively. Follow up data (e.g. bleeding episode, adverse event, status of administration, etc) was recorded for every 6 month period.
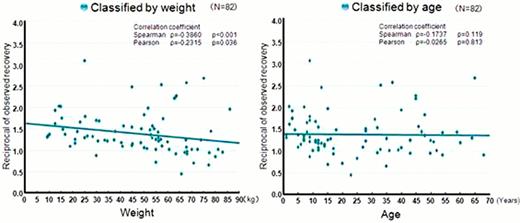
319 patients including 32 PUPs were registered from 173 institutions during 2 years. 177 patients including 14 PUPs were included in this interim analysis as the data cutoff was Jan 2012. Duration of the observation was 6 to 12 months (PUPs: 6 to 18 months). Disease severity was 52.0% (severe), 28.8% (moderate), 15.8% (mild) and 3.4% (unknown). Total frequency of the administration was 1 to 240 infusions (median: 41 infusions). Some patients received more than one Benefix regimen (e.g. prophylaxis + on demand) and assessment was conducted by each regimen (prophylaxis: 98, on demand: 55, hemostasis during surgery: 13, others: 38). Total 512 bleeding episodes (spontaneous: 440, traumatic: 66, unknown: 6) were reported from 55 patients who received on demand therapy. An average of 2.2 times infusions was needed for hemostasis. The average dose was 41IU/kg. Regarding 98 patients who received prophylactic regimen, the most common regimen was twice-weekly administration (62.4%) and its average dose was 40 IU/kg. Analyzed by age, the average dose was 44 IU/kg and 35 IU/kg for <15 years old and ≥ 15 years old, respectively. No bleeding was observed in 68.3% (by age: 75.0% of <15 years old, 62.8% of ≥ 15 years old). Average frequency of bleeding was 3.8 times (median: 2, max: 45) in 6 months period. Factor IX levels were measured in 82 patients after first dose (median dose: 44IU/kg, range: 17 to 120 IU/kg). Median reciprocal of observed recovery was 1.28 (min-max). Weak negative correlation (r2 = 0.463, p=0.036) was observed between weight and recovery. A correlation was not seen between the recovery and age.
Adverse drug reaction was observed 2.3% of patients (2 headaches, 1 vertigo, 1 dyspnea, 1 nausea, 1 urticaria and 1 itching of injection site). No patients developed inhibitor related to nonacog alfa.
Nonacog alfa was safe and effective on both prophylactic and on demand regimen in clinical practice. 68.3 % of no bleeding rate was comparable to plasma-derived factor IX. The variability in recovery was consistent with what has been reported by others. This surveillance study is still ongoing and final result will be obtained in year 2014.
Suzuki:Pfizer Japan Inc.: Research Funding. Fukutake:Pfizer Japan Inc.: Research Funding. Amano:Pfizer Japan Inc.: Research Funding. Hanabusa:Pfizer Japan Inc.: Research Funding. Taki:Pfizer Japan Inc.: Research Funding. Matsushita:Pfizer Japan Inc.: Research Funding. Shima:Pfizer Japan Inc.: Research Funding. Sakai:Pfizer Japan Inc.: Research Funding. Iizuka:Pfizer Japan Inc: Employment. Shibasaki:Pfizer Japan Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.