Abstract
Abstract 3432
Patients who undergo on-pump coronary artery bypass graft surgery (CABG) are at an increased risk of bleeding due physiologic, mechanical and pharmacologic disruption of hemostasis. The use of topically applied antifibrinolytic agents has been explored as a blood-conservation adjunct to reduce post-operative bleeding in cardiac surgery. Analysis of pooled results in a meta-analysis published by Abrishami et al. in 2009 showed that a significant reduction in blood loss could be achieved utilizing topical tranexamic acid in the absence of concurrent intravenous antifibrinolytic agents. However, current blood conservation clinical practice guidelines recommend the use of intravenous lysine analogues to limit peri-operative blood loss.
Presently, there is no published prospective data evaluating blood loss in CABG patients who have received intravenous tranexamic acid, plus topical tranexamic acid versus placebo. Our study was designed to determine whether combined intravenous and topical application of tranexamic acid would reduce post-CABG blood loss in low-risk bypass surgery candidates. A sample calculation based on pilot study data analysis concluded that a minimum total of 74 patients was required, allowing 80% power to detect a 200mL difference in total blood loss (Type I error = 0.05) between groups.
In our prospective, double-blind, randomized controlled trial, patients enrolled were randomly assigned to receive an intra-operative cardiac bath of either normal saline or tranexamic acid solution prior to sternotomy closure. All participants received intravenous tranexamic acid prior to the initiation of circulatory bypass. Research subject participation ended upon transfer out of the intensive care unit. Primary outcomes included the total volume of chest tube blood loss and number of transfusions administered following CABG. Secondary outcomes included chest tube loss volumes at 6 hours and 12 hours.
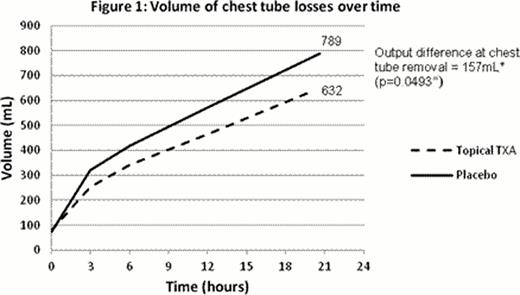
Between December 1, 2011 and April 30, 2012, a total of 41 patients who underwent randomization were included in final data analysis. Baseline characteristics of patients enrolled in both groups were similar. Post-CABG losses at chest tube removal were found to be significantly reduced in the patients who received topical tranexamic acid. Mean chest tube loss in the placebo group (n=18) was 789mL versus 632mL in the topical tranexamic acid group (n=23), with a difference of 157mL (Figure 1, p value of student t-test 0.0493). A significantly greater proportion of patients in the placebo group were found to bleed more than 700mL at chest tube removal, in comparison with the topical tranexamic acid group (72.2% versus 30.4%, p value of chi-squared test 0.0079). Chest tubes remained in situ for a mean duration of 20 hours and 19 hours, respectively. None of the patients received post-operative red cell transfusions. One patient, randomized to the topical tranexamic acid group, received a single dose each of platelets and cryoprecipitate post-operatively. The application of topical tranexamic acid did not significantly reduce blood loss at 6 hours and 12 hours, with a difference of 76mL (p=0.1876) and 106mL (p=0.0930) respectively.
The application of topical tranexamic acid reduces post-operative blood loss in patients undergoing on-pump CABG who have received intravenous tranexamic acid. There was no difference in blood product use between groups. Further studies must be carried out to investigate long-term outcomes of this practice and its applicability in high-risk cardiac surgery patients. (ClinicalTrials.gov number, NCT01519245.)
Off Label Use: Intravenous and topical application of tranexamic acid in cardiac surgery.
Author notes
Asterisk with author names denotes non-ASH members.