Abstract
Abstract 3483
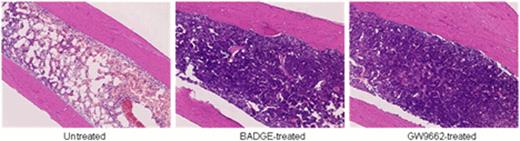
In aplastic anemia (AA), the marrow is not “empty” but replaced by fat; the increase in adipocytes number and size is most obvious on three dimensional reconstructions of the marrow in human and murine (Takaku T, Blood. 2008). A reciprocal relationship exists between adipogenesis and osteogenesis, and osteoblasts constitute the hematopoietic niche and play an active role in the regulation of stem cells and progenitors. Fat in the marrow has been considered an epiphenomenon in AA. However, a recent report suggested that bone marrow (BM) adipocytes negatively regulated hematopoiesis in mouse models (Naveiras O, Nature. 2009). Peroxisome proliferator-activated receptor-g (PPAR-g) is a key transcription factor for adipogenesis, and blocking PPAR-g signaling inhibited adipogenesis in vitro (Wright HM, J Biol Chem. 2000). To examine the role of BM adipocytes, we investigated the effects of PPAR-g antagonists, bisphenol A diglycidyl ether (BADGE, 30 mg/kg/day) and GW9662 (1 mg/kg/day), on hematopoiesis in a mouse model of immune-mediated BM failure (Chen J, J Immunol. 2007). We induced BM failure by infusion of lymph node (LN) cells from C57BL/6 mice into sublethally irradiated C.B10-H2(b)/LilMcd (C.B10) recipient mice that were matched at major histocompatibility antigens but differed in multiple minor histocompatibility antigens. In adaptation of the “runt” disease model, mice uniformly develop progressive and fatal pancytopenia, closely resembling human BM failure, without other evidence of graft-versus-host disease. We treated recipient mice with BADGE, GW9662, or control vehicle from day -1 to day 14. On day 14, mice were sacrificed and evaluated by peripheral blood (PB) cell counting and BM cellularity, as well as morphology of marrow adipocytes. Mice in the BADGE- and GW9662-treated groups showed higher numbers of leukocytes, neutrophils, and platelets in PB and higher total nucleated cells and Lin- Sca1+ c-kit+ stem cells in BM than did animals in the control group. Both confocal microscopic imaging and hematoxylin and eosin staining of BM also showed significantly higher numbers of nucleated cells and many fewer and smaller adipocytes in the treated groups (Figure 1). We also investigated dose response of BADGE in the treatment of AA mice. Low dose of BADGE (15 mg/kg/day) had no effect while high dose of BADGE (60 mg/kg/day) seemed to have no extra benefit for the BM hematopoiesis compared with the medium dose (30 mg/kg/day). However, we also noted in PPAR-g antagonist-treated groups that there was significantly less CD8+ T cell infiltration of BM, as determined by flow cytometry. We speculated that PPAR- g antagonists might also negatively affect activation of cytotoxic T cells. By magnetic beads-based multiplex assay, we found the concentrations of inflammation-related cytokines in the plasma, including Interleukin-6, tumor necrosis factor alpha, monocyte chemotactic protein-1 were markedly decreased in PPAR- g antagonist-treated groups. When we performed PCR arrays focusing on adipogenesis and inflammasome pathways, we found that expression of adipogenesis genes was greatly decreased in the treated groups, including Agt (−149 folds), Cebpa (−4.7 folds), Acacb (−11.7 folds), Fabp4 (−3.2 folds), Adig (−14.2 folds), and Bmp2 (−12.9 folds). The expression of inflammation- or inflammasome-related genes including Nlrc4 (−11.3 folds), Mapk12 (−4.8 folds), Ptgs2 (−8.7 folds), and Rela (−5.9 folds) was also decreased while apoptosis inhibitor genes including Xiap (+17.5 folds), Mapk1 (+6.6 folds), and Bcl2l1 (+3.9 folds) were increased in the treated groups. In vitro, BADGE and GW9662 inhibited activation and proliferation of T cells stimulated with anti-CD3/CD28 or phorbol myristate acetate/ionomycin. These data suggested that BADGE and GW9662 inhibition was not specific for adipogenesis but affected T cell activation. Indeed, PPAR-g antagonists failed to ameliorate pancytopenia and BM hypoplasia in the mice exposed to either a lethal or sublethal dose of total body irradiation. PPAR-g antagonists may act to attenuate murine immune mediated marrow failure by mechanism of inhibition of T-cell activation.
Histology of femurs from untreated bone marrow failure mice and PPAR-g antagonists treated mice. Both BADGE and GW9662 inhibited adipogenesis and increased cellularity in the bone marrow of AA mice.
Histology of femurs from untreated bone marrow failure mice and PPAR-g antagonists treated mice. Both BADGE and GW9662 inhibited adipogenesis and increased cellularity in the bone marrow of AA mice.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.