Abstract
Abstract 3495
WM is a rare malignant B-cell disorder characterized by lymphoplasmacytic infiltration of the bone marrow (BM) and hypersecretion of monoclonal IgM. IgMMGUS is an asymptomatic condition characterized by the presence of a serum monoclonal IgM protein and bone marrow infiltration < 10%. WM (symptomatic and indolent) and IgMMGUS can be identified based on two main features, the bone marrow infiltration and the existence of signs and symptoms. The biological and genetic characteristics of both conditions need to be explored.
Our study aims to highlight the different expression profiles between WM and IgMMGUS comparing CD19+ as well as CD138+ cells. We have investigated patients with WM (n =21) and patients affected by IgMMGUS (n=10). BM CD19+ and BM CD138+ cells were isolated from 21 WM patients, while BM CD19+ and BM CD138+ were isolated from 10 and 4 IgMMGUS patients, respectively. Microarray analysis was performed using Affymetrix GeneChip Human Genome U133 Plus 2.0 Array. Data was preprocessed using Robust Multi-Array Average (RMA) software. Differential expression analysis was performed using Significant Analysis of Microarrays (SAM). Genes showing a q value lower than 5% and an absolute fold change greater than 2 were selected for further clustering analysis which was performed applying complete linkage hierarchical agglomerative clustering on Euclidean pairwise distances between genes.
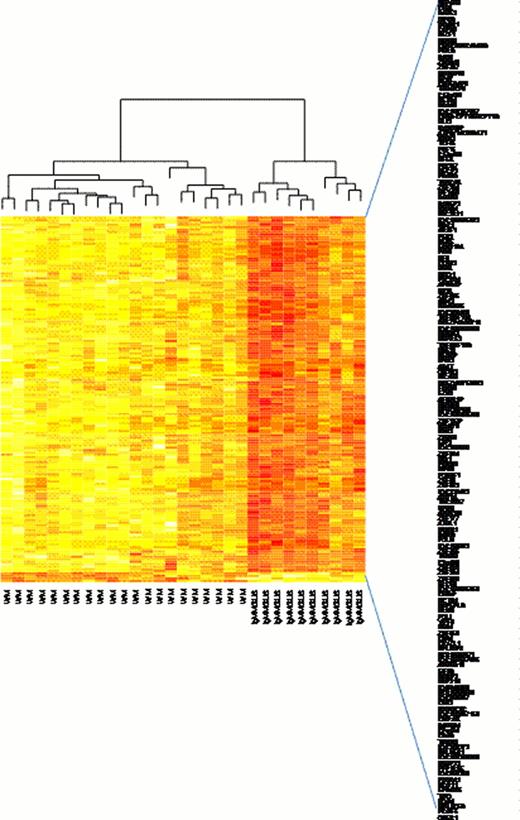
Microarray of WM vs IgMMGUS CD19+ cells has highlighted 151 differently expressed genes (Fig. 1). Among them we have found 33 genes involved in the regulation of transcription which were significantly overexpressed in WM vs. IgMMGUS. BM WM CD19+ cells overexpressed the following 14 Zinc Finger Protein (ZNF) genes: ZBTB40, ZNF83, ZNF137P, ZNF177, ZNF224, ZNF264, ZNF320, ZNF395, ZNF514, ZNF532, ZNF623, ZNF767, ZNF785, ZNF850.
Other genes acting as regulators of transcription overexpressed in WM B cells were: HIF1AN, BHLHE41, EZH1, CCNL2, TCFL5, BLZF1, CIITA, PER2, MECP2, PRDM2, and ELP2. In particular, HIF1AN belongs to the PI3K/Akt/mTOR pathway, ELP2 is involved in the JAK/STAT process, and BHLHE41 and PER2 are included in the cicardian clock showing that these different biological mechanisms differently develop in WM with respect to IgMMGUS.
TNFRSF10A, MAP4K4, TNFRSF10B, WNK1, DUSP22, ITPKB genes were overexpressed in WM B cells showing the involvement of Akt and MAPK signaling pathways which can play an important role in WM cells as they regulate several biological processes including cell growth, differentiation, survival, migration and metabolism.
Gene expression profiling across CD138+ cells have demonstrated 43 differently expressed genes between WM vs. IgMMGUS (Fig. 2). MS4A1, BANK1 genes were overexpressed with high fold changes (FC) of 11.6 and 9.4, respectively, as well as GPR183, SWAP70 which were significantly overexpressed in WM vs. IGMMGUS, both genes showing a FC of 5. These results suggest that B cell activation and immune response are biological processes which act differently in WM compared to IgMMGUS.
RALGPS2(FC=4), PLEKHG1 (FC=10) and ARHGAP24 (FC=4) genes mediating GTPase regulator activity of signal transduction were overexpressed in WM. ARHGAP24 could also be involved in the modulation of angiogenesis.
FCRL2 and FCRLA genes involved in cell-cell signaling and cell differentiation, were significantly overexpressed in WM vs. IgMMGUS CD138+ with a fold change of 4.6 and 9, respectively. Again, the immune response seems to be a biological mechanism involved in WM CD138+ cells as CD79B (FC=5) and HLA-DOA (FC=4) genes were overexpressed in WM in respect to IgMMGUS.
These differences may reflect varied immune mechanisms in the two disorders. In conclusion, the regulation of transcription, PI3K/Akt/mTOR and MAPK signaling pathways are the most relevant gene ontology biological processes occurring in CD19+ cells, while immune response, cell activation and signaling processes developing in CD138+ cells mainly distinguish WM and IgMMGUS.
Future studies of the biological role of the genes differently expressed in WM vs IgMMGUS could clarify the pathogenetic processes underlying IgMMGUS and WM. The understanding of the molecular mechanisms leading to the progression of IgMMGUS to WM could help the identification of IgMMGUS patients who are at high risk for progression to WM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.