Abstract
Abstract 3533
microRNAs (miRNAs) control many developmental and physiological processes. However, it is assumed to work as a fine tuner in cell fate determination, which has been shown to be regulated by transcription factors.
Here, we challenge this canonical notion.
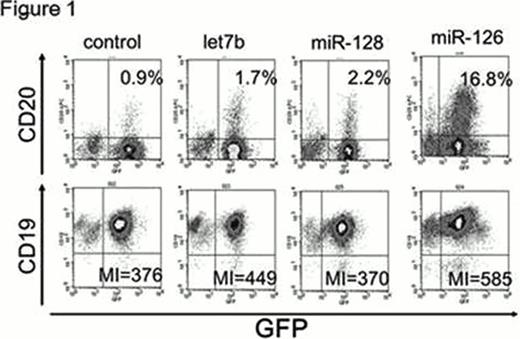
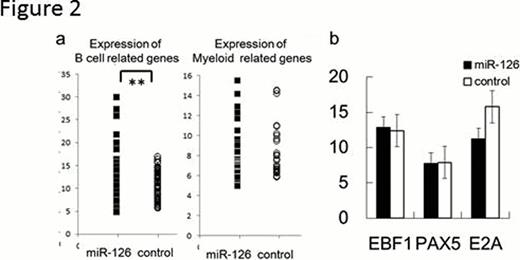
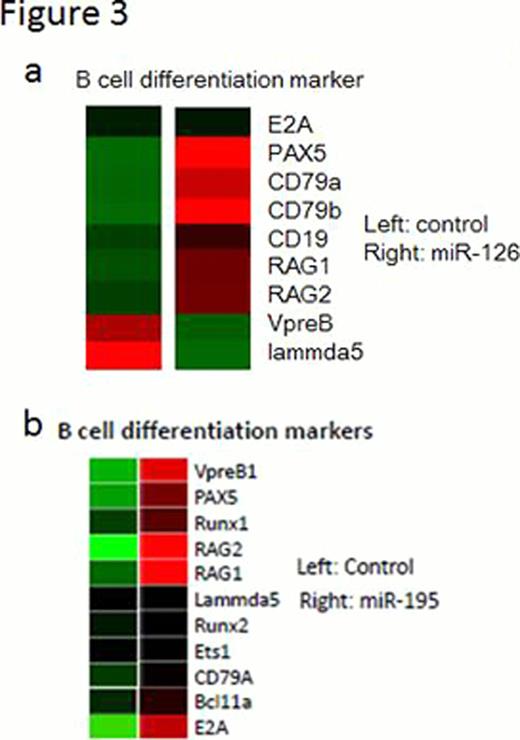
miR-126 is downregulated in MLL-AF4 ALL, biphenotypic leukemia expressing both B cell and myeloid markers, compared with other types of ALLs. CD19 and CD20, B cell differentiation markers, were upregulated when miR-126 is reexpressed in MLL-AF4 ALLs (Figure 1). Interestingly, we found that miR-126, in leukemic cells, induces B cell differentiation (Figure 2a) without changing the expression of E2A, EBF1, or PAX5, (Figure 2b) which are assumed to be critical regulators of B cell development indicating that miR-126 induced B cell differentiation independently of transcriptional factors, such as PAX5, E2A, and EBF1. To test the hypothesis, we tried to rescue block of B cell differentiation in EBF1 knock-out hematopoietic progenitor cells (EBF1 KO HPCs) by exogenous expression of miR-126. As a result, significant upregulation of B cell markers, such as B220, RAG1/2, and CD79a/b was induced by miR-126 in EBF1 KO HPCs which show complete block at pre-pro B cell stage.(Figure 3a) Moreover, miR-126 increased cell proliferation, indicating EBF1 KO HPCs differentiate into large preB cells. Moreover, miR-195, which is upregulated toward pro B cells then downregulated in B cell differentiation, also can partially rescue block of B cell differentiation in EBF1 KO HPCs. (Figure 3b) The block in B lymphopoiesis imposed by the absence of E2A or Pax5 can be overcome by exogenous expression of EBF1. Conversely E2A or Pax5 failed to compensate the effect of EBF1. Thereafter miR-126 and miR-195 potentiates to play more roles than E2A or Pax5 in this system and act as more than a fine tuner in B cell differentiation. Our results elucidate a novel mechanism for the regulation of cell fate independent of transcriptional factor, establish an important role for miRNAs in mammalian lineage specification, and suggest that miRNA could be a new possible therapeutic target for dedifferentiation induced by deregulation of transcriptional factors in ALL, which calls for further studies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.