Abstract
Abstract 3694
Based on published favorable Phase 2 data in patients with relapsed/refractory non Hodgkin Lymphoma (NHL)(Wiernik, 2008), the Italian Drug Agency (AIFA) granted the nulla osta to patients with NHL who were ineligible to receive more intensive curative treatments, which allowed off-label treatment with lenalidomide on a case by case basis. From April 2008 to December 2010, 180 NHL patients, most of them diagnosed with Diffuse Large B Cell Lymphoma (DLBCL) and Mantle Cell Lymphoma (MCL), were treated with lenalidomide. All patients were tracked in an ad hoc database, in order to ensure compliance with the Risk Management Plan (RMP) already in place for the authorized use of lenalidomide in the multiple myeloma indication.
Data collected from this cohort of NHL patients were retrospectively analyzed, with the aim to assess the safety and efficacy of lenalidomide administered to a heavily pretreated patient population in the context of routine clinical practice. To our knowledge, this is the largest NHL patient cohort treated with lenalidomide outside of a clinical trial.
Data on 157 patients from 44 Italian sites have been collected and analyzed to date. Patient demographics and disease characteristics were as follows: median age 70 years (range 19–92); median number of prior treatments 3 (range 1–17); 94% of patients previously treated with rituximab. The lymphoma subtypes included DLBCL in 44%, MCL in 35%, Follicular Lymphoma (FL) in 9% and Transformed Lymphoma (TL) in 6% of cases. As expected, 62% of patients had Stage IV disease, in keeping with the highly unfavorable characteristics of a heavily pretreated patient population. In the majority of treatment cycles, lenalidomide was administered at a dose of 25 mg (61%) and in combination (58%). Analyzing all cycles, dexametasone was the preferred drug combined with lenalidomide (35%), while rituximab was added in 10%. Median number of administered lenalidomide cycles was 3 (range 1–27). According to the International Workshop on Lymphoma Response Criteria 42 % of patients responded, with 18% CR + CRu and 24 % PR. The breakdown of overall responses according to the different lymphoma subtypes shows: a rate of 45%, 39%, 54%, and 33% in DLBCL, MCL, FL, and TL patients, respectively. Median duration of response was 7.8 months overall, 14.2 months specifically for patients who achieved CR/CRu.
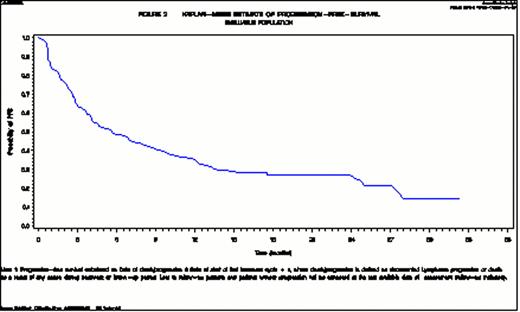
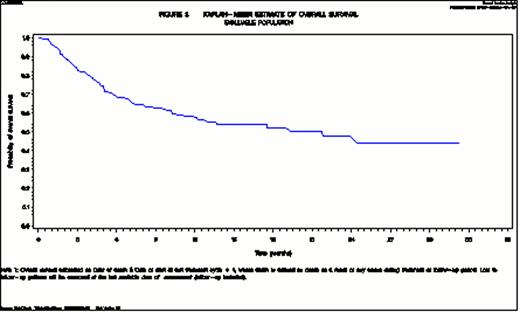
Median TTP and PFS are 7.8 and 5.7 months (Figure 1) respectively, and median OS 21.8 months (Figure 2). The safety and tolerability profile of lenalidomide appears to be comparable to what was already known from published clinical trial results in NHL (Wiernik et al., JCO 2008, Habermann et al., BJH 2009, Witzig et al., Ann of Onc 2011, Zaja et al., Haematol 2011, Zinzani et al., Clin Lymph Myel Leuk 2011, Wang et al., Lancet Onc 2012). No secondary neoplasms have been reported to date.
In conclusion, with the limitations of a retrospective data collection, this analysis provides intriguing results on the use of an immunomodulatory drug such as lenalidomide in a large cohort of heavily pretreated NHL patients, who have limited therapeutic options or who are ineligible for more intensive curative treatments. In this very unfavorable patient setting, lenalidomide has shown incouraging response rates as well as PFS and OS values, suggesting anti-lymphoma activity.
Off Label Use: Lenalidomide (Revlimid) has indication in the treatment of multiple myeloma. Based on several published phase II study we have used this drug, granted by the nulla osta of the Italian Drug Agency (AIFA), as off-label therapy of non Hodgkin lymphomas regardless of histology. The aim of this retrospective study was to assess the safety and efficay of this drug in a cohort of patients heavily pretreated.
Author notes
Asterisk with author names denotes non-ASH members.