Abstract
Abstract 3776
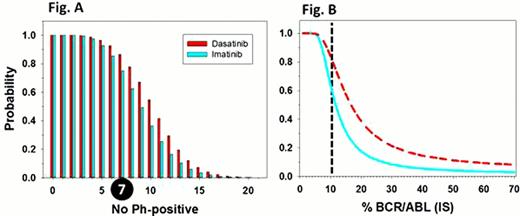
Existing therapeutic guidelines for CML are centered on the establishment of cytogenetic/molecular disease response milestones for assessing prognosis and recommendations for treatment decisions. Categorical risk classifications have been established from the analysis of large cohorts of patients on trial and thousands of cytogenetic and molecular analysis. While such analysis can be regarded as robust, little attention has been given to assessing the reliability of correctly assigning a patient to a given disease response category based on a single bone marrow cytogenetic analysis or quantification of BCR/ABL by PCR at a specific time point. More recently, the achievement of a cytogenetic response of ≤ 35% Ph (MCR) or BCR/ABL ≤ 10%IS at three months on first line treatment with imatinib or second generation TKIs has been shown to predict for significant differences in subsequent MMR, PFS,OS, and its adoption as a new milestone has been proposed. In the DASISION trial, the rate of MCR at 3 months for imatinib and dasatinib was 67% and 81%, and BCR/ABL ≤ 10%IS 64% and 84% respectively. The rate of BCR/ABL ≤ 10%IS at 3 months in the ENESTnd trial was 77% and 91% for imatinib and nilotinib respectively. The posterior probability of a patient having achieved an MCR at three months based on the analysis of 20 metaphases (ie. ≤ 7/20 Ph-positive) based on a Bayesian analysis is shown in Fig. A Calculations were based on the apriori probabilities of achieving an MCR at three months of 67% and 81% for imatinib and dasatinib. Conditional probabilities (probability of achieving ≤ 7/20 Ph+ if in MCR or > 7/20 Ph+ if not in MCR) were derived from the cumulative Poisson distribution. Bayesian analysis of the posterior probability of BCR/ABL ≤ 10%IS is shown in Fig. B using apriori probabilities of 64% and 84% for imatinib and dasatinib, and conditional probabilities based on normal distribution of PCR results with a coefficient of variance (CV) of 40%. The analysis demonstrates that the probability of incorrectly assigning a patient as having failed to reach an MCR is surprisingly high for results of > 7/20 Ph+ metaphases (ie. 20% for 12/20 Ph+ for dasatinib), or BCR/ABL ≤ 10%IS (ie. 20% for BCR/ABL ∼ 35%IS for dasatinib). These results signal the need for caution in assigning the categorization of response and risk in individual patients to those of much larger cohorts and highlight the need to acknowledge current limitations in therapeutic guidelines. Accuracy of cytogenetic testing can only be improved by increasing the number of metaphases analyzed, which is often not feasible. With lower achievable PCR assay CVs the accuracy of PCR can exceed that possible with cytogenetic analysis. Details of the analysis and comparative ROC curves will be presented.
Laneuville:Novartis: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau.