Abstract
Abstract 3856
Immunological responses play an important role in the pathogenesis and progression of myelodysplastic syndromes (MDS). Several studies have confirmed that immune dysregulation in MDS may play a critical role in the initiation and progression of the dysplastic clone. We have already shown the importance of expanded Tregs in high risk MDS and its reverse correlation with the number of Th17 cells in low risk disease. However, the potential role of dendritic cells (DCs) in this immune dysregulation and in the expansion of Tregs in MDS is not fully understood. DCs are professional antigen presenting cells (APCs) and potent stimulators of T cells through (cross-)presentation of antigens via MHC class I and II molecules to CD8+ cytotoxic T lymphocytes (CTL) and CD4+ T helper (Th) cells, respectively. DCs are also important in the development of specific anti-tumor T-cell responses. Some subtypes of DCs also play an important role in the expansion of regulatory T cells (Tregs) and induce immune-suppression and editing. There are 3 main subpopulations of DCs in human: plasmacytoid DCs (CD303+ cells) and two types of myeloid DCs (CD1c+ and CD141+ cells).
The aim of this study was to investigate the frequency of different DC subsets in the bone marrow and peripheral blood of MDS patients.
Twelve peripheral blood and 26 bone marrow samples from MDS patients and 11 peripheral blood and 4 bone marrow samples from healthy donors (HDs) were studied. Three different DC subsets were investigated by flow-cytometry: plasmacytoid DC (Lineage−, HLA-DR+, CD303+), myeloid DC 1 (Lineage−, HLA-DR+, CD1c+) and myeloid DC 2 (Lineage−, HLA-DR+, CD141hi).
Decreased frequency of DCs in peripheral blood compared to BM
Frequencies of all DC subsets were significantly lower in patients' peripheral blood compared to patients' bone marrow (CD1c+ DC 2.56×102 v 7.02×102 and 0.06% v 0.33%, p=0.001; CD141+ DC 4.18×103 v 6.04×103 and 0.44% v 2.44%, p=0.005; CD303+ DC 0.06×102 v 1.07×102 and 0.01% v 0.13%, p=0.045).
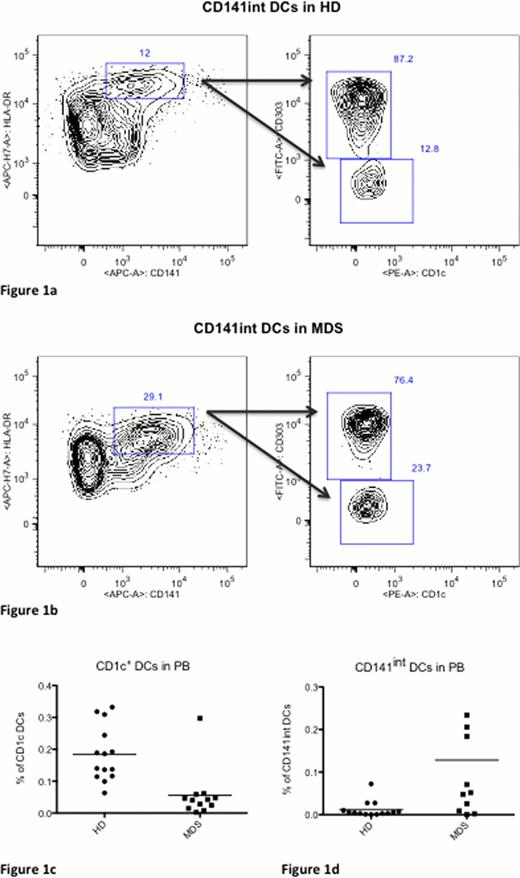
The percentage of the CD1c+ DC subset was significantly reduced in the peripheral blood of MDS patients compared to HD (1.46×103 v 0.26×103, 0.18% v 0.05%, p=0.001) (figure 1). Interestingly, we have noticed an additional subset of DCs, which was not described before. The definition of this subset was based on intermediate expression of CD141 compared to previously described myeloid DCs. The CD141int subset was the only DC subset that was increased in peripheral blood of MDS patients compared to HD (0.41×102 v 2.30×102, 0.01% v 0.13%, p=0.019). Both CD1c+ and CD141int DC subsets were increased in the bone marrow of MDS patients compared to healthy donors. However, these differences were not statistically significant.
Although the decreased number of circulating DCs is reported in MDS, our data suggest that this decrease is more profound in the peripheral blood compared to BM. We have also shown that this decrease is mainly in CD1c+ myeloid DCs. In this study, we describe two subsets of CD141 DCs, CD141hi and CD141int. The CD141int subpopulation is the only DC subset that is increased in MDS patients compared to HD.
Our data suggest that DCs from peripheral blood may migrate to the bone marrow in response to an aberrant BM microenvironment in MDS.
Nevertheless, the pattern of decrease in DC numbers is not uniform and CD141int DCs are in fact increased. It is already known that CD141+ DCs, play an important role in the induction of Tregs in an IL-10 dependent manner. We have also reported an increased number of Tregs in high-risk MDS, which correlates, with higher risk of progression toward AML. Our data suggest that CD141+ DCs may play a role in the expansion of Tregs in MDS. However, the exact function of this subset of DCs in MDS and their effect on CD4+ T-cells polarization needs to be investigated in more details.
Gating strategy for CD141int DCs in (A) HD and (B) healthy donors. Cells were gated from HLA-DR+Lin− cells. The CD141int cells were considered to be CD303− and CD1c−. (C) The frequency of CD1c+ cells was significantly reduced in MDS patients (p= 0.001) and (D) CD141int DCs were significantly increased in MDS patients (p=0.019). ADDIN EN.REFLIST
Gating strategy for CD141int DCs in (A) HD and (B) healthy donors. Cells were gated from HLA-DR+Lin− cells. The CD141int cells were considered to be CD303− and CD1c−. (C) The frequency of CD1c+ cells was significantly reduced in MDS patients (p= 0.001) and (D) CD141int DCs were significantly increased in MDS patients (p=0.019). ADDIN EN.REFLIST
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.