Abstract
Abstract 3960
Overwhelming osteoclast (OCL) activation plays a central role in multiple myeloma (MM)-related bone disease. It is well known that in MM, OCL differentiation and final maturation rely upon RANKL stimulation by bone marrow mesenchymal cells and osteoblasts (OBLs). The clinical relevance of this pathway has been recently underlined by the anti-resorptive activity mediated by the mAb Denosumab.
Since the discovery of microRNAs (miRNAs), findings on their role on intracellular pathway control have undergone a tremendous progress suggesting their potential use as a therapeutic tool against specific targets.
Based on these premises, we aimed to identify miRNAs that can be relevant for the management of MM-related bone disease.
Among miRNAs deregulated in MM, miR-29 family has been implicated in bone pathophysiology. Indeed, miR-29 family promotes OBL generation, while miR-29b might interfere with OCL differentiation and function by targeting RANKL axis and Metalloproteinase II (MMP2), that confers the property to degradate type I collagens.
Therefore, we studied whether miR-29b can have a detrimental effect for terminally differentiated OCLs.
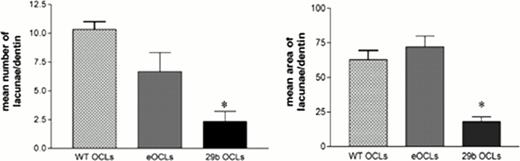
We generated OCLs in vitro upon RANKL/MCSF stimulation starting from CD14+ hematopoietic precursors and found that miR-29b levels decline along OCL differentiation, reaching statistical significance as compared with its precursors (p=0,013). These data suggested that terminally differentiated OCLs do not require miR-29b. In order to assess whether miR-29b reconstitution could affect OCL activity, we transduced OCLs with a miR-29b coding sequence- carrying lentiviral vector (29b OCLs) or an empty lentiviral vector (eOCLs) and evaluated them in morphological and functional assays. As we expected, 29b OCLs showed a faint and irregular expression of tartrate acid phosphatase (TRAcP), which is highly and uniformously distributed within WT/eOCLs. Furthermore, when 29b OCLs were seeded on dentin, generation of lacunae on dentin surface, which recapitulates bone resorption, was significantly reduced as compared with WT/eOCLs (see Figure). According to these results, we measured the release of type I collagen fragments by OCLs and found that degradation of type I collagens was significantly impaired in 29b OCLs, suggesting that constitutive expression of miR-29b strongly antagonizes OCL differentiation and bone lytic functions.
In order to reproduce the features of MM related bone disease, we co-cultured OCLs with RPMI-8226 MM cells, which are able to stimulate WT/eOCLs to generate lacunae on dentin slices in the absence of exogenous RANKL/MCSF. We observed that 29b OCLs failed to generate comparable numbers and areas of the pits in presence of MM cells (p=0,035/p=0,04).
To support these functional data and ascertain the effects of miR-29b on specific pathways, we evaluated the expression of SP1 and NFATc-1, which are relevant transcription factors for OCL differentiation and work through RANK-L axis. We found that both factors are down-modulated in 29b OCLs as compared to WT/eOCLs. Down modulation was observed also for MMP2, thus mirroring the reduced capability to lyse type I collagens.
Overall, our data indicate that miR-29b impairs OCL differentiation and function even in presence of robust stimuli such as RANKL and MCSF. We provided molecular support to these functional data, showing that SP1 and NFATc are down modulated in presence of miR-29b as well as MMP2, which is involved in collagen degradation. Intriguingly, MM cells, which represent a strong pro-osteoclastic factor, were not able to revert OCL functional impairment. We believe that these relevant preclinical findings allow to propose miR-29b mimics as a suitable and attractive candidate to be developed as a novel and innovative treatment of MM-related bone disease.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.