Abstract
Abstract 4050
Multiple myeloma (MM) patients who are dual refractory to or intolerant of bortezomib (Bor) and lenalidomide (Len) have a poor prognosis. Kumar et al demonstrated a progression free survival of 5 months (mo) and overall survival (OS) of 9 mo in this population (Kumar et al, Leukemia, 2011). However, they did not define demographics of this population in detail, and none of their patients received next-generation drugs carfilzomib (Carf) or pomalidomide (Pom).
We aimed to define the unique demographic characteristics of patients with MM who failed both Bor and Len in detail, and to ascertain the effects of treatment with Carf or Pom on survival of this patient cohort.
Sixty-five MM patients followed at Washington University who became dual refractory/intolerant of Bor and Len were identified by chart review from January 2007 to May 2012. Detailed demographics were summarized by descriptive statistics. We performed univariate and multivariate analyses by Cox regression to identify predictors associated with OS in this population. OS was estimated using Kaplan-Meier method and comparisons were made by Wilcoxon test.
The median age at the diagnosis of MM was 58 years (range 34–77). Sixty-three percent (N=41) were male, 78% (N=51) Caucasian, 20% (N=13) African American, and 2% (N=1) Asian. We defined the date when patients became dual refractory/intolerant of Bor and Len as time zero (T0). The median age at T0 was 62 years (range 39–79). The median interval from diagnosis to T0 was 39 mo (range 2–160).
At T0, 28% (18/64) of patients had poor bone marrow reserve (neutrophils < 1000/mm3 or platelets < 75,000/mm3). Twenty-four percent (15/62) had high-risk cytogenetics (hypodiploidy, del 13, del 17p, t(4;14), or complex cytogenetics). Seventy-two percent (47/65) had advanced lytic bone disease and 14% (9/65) had extramedullary disease. Thirty-four percent (20/58) had oligosecretory myeloma (serum M-protein < 1g/dL and urine M-protein < 200mg/24hrs) at T0, compared to only 9% (6/65) at diagnosis. Twenty-nine percent (15/52) were stage 3 by International Staging System at T0.
Patients received a median of 5 lines of therapies prior to T0. In addition to Bor and Len, other treatments included: thalidomide (63%), anthracyclines (46%), and alkylating agents (37%). Forty-eight (74%) patients had autologous stem cell transplantation (SCT) prior to T0. Patients received a median of 2 lines of therapies after T0, including Carf (17%), Pom (23%), anthracyclines (14%), and alkylating agents (45%). Nine (14%) patients underwent SCT after T0, 5 autologous and 4 allogeneic.
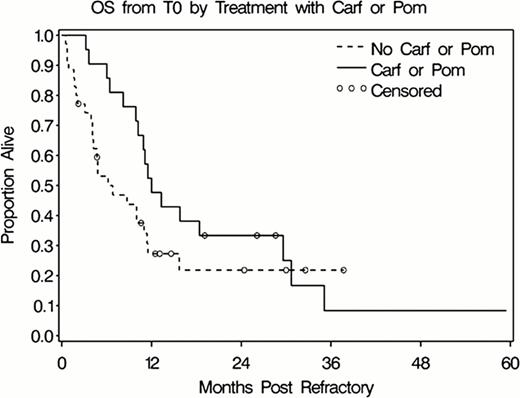
The median OS from T0 was 10.2 mo (range 0.5–59.4). To study the effect of Carf/Pom on OS we excluded patients who received SCT after T0 since a disproportionate number of patients in the non-Car/Pom arm underwent SCT after T0. Patients who received Carf or Pom after T0 had an improved 12-mo OS compared to those who did not (47.6% vs 27.3%; p=0.02 by Wilcoxon Test), (Figure 1). Multivariate analyses showed that low hemoglobin, advanced lytic lesions, treatment with VRD prior to T0, and lack of treatment with Carf or Pom after T0 were all independent predictors for poor survival in this population (Table 1).
Patients who are dual refractory to or intolerant of Bor and Len have a high incidence of poor marrow reserve, high-risk cytogenetics, extramedullary disease, and oligosecretory phenotype. This information is crucial for designing clinical trials for this population of patients. Next generation agents Carf and Pom after T0 are associated with approximately 50% reduction in risk of death. Prospective trials are needed for confirmation.
Predictors of Poor Survival by Multivariate Logistic Regression
| Variable . | Hazard Ratio . | 95% Confidence Interval . | p Value . |
|---|---|---|---|
| Carf or Pom (Yes vs. No) | 0.52 | 0.27–0.99 | 0.047 |
| Advanced lytic lesions (Yes vs. No) | 2.52 | 1.20–5.29 | 0.014 |
| VRD prior to T0 (Yes vs. No) | 2.28 | 1.19–4.37 | 0.013 |
| Hemoglobin (g/dL) (per unit increase) | 0.71 | 0.58–0.86 | 0.0004 |
| Variable . | Hazard Ratio . | 95% Confidence Interval . | p Value . |
|---|---|---|---|
| Carf or Pom (Yes vs. No) | 0.52 | 0.27–0.99 | 0.047 |
| Advanced lytic lesions (Yes vs. No) | 2.52 | 1.20–5.29 | 0.014 |
| VRD prior to T0 (Yes vs. No) | 2.28 | 1.19–4.37 | 0.013 |
| Hemoglobin (g/dL) (per unit increase) | 0.71 | 0.58–0.86 | 0.0004 |
Off Label Use: Here we discuss the use of pomalidomide in the treatment of relapsed/refractory multiple myeloma. Vij:Celgene, Millennium and Teva: Speakers Bureau; Onyx and Celgene: Research Funding; Onyx: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.