Abstract
Abstract 4057
Combinations of older drugs and novel agents are constantly improving the outcome of chemotherapy in AL amyloidosis. Bendamustine has demonstrated activity in multiple myeloma and Waldenström macroglobulinemia. In the present study we evaluated the safety and efficacy of Bendamustine and prednisone (BeP) in 36 patients with AL amyloidosis from two European referral centers, the Amyloidosis Center (Heidelberg, Germany) and the Amyloidosis Research and Treatment Center (Pavia, Italy).
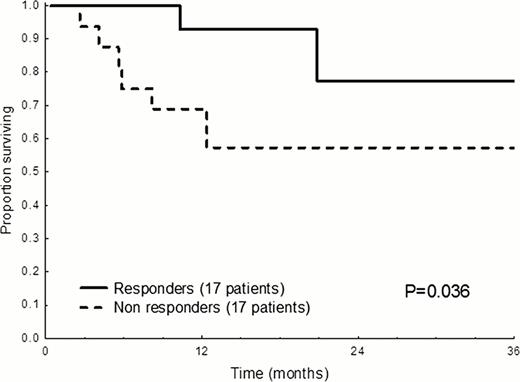
The databases of the two centers were systematically searched for patients with AL amyloidosis treated with BeP. Nineteen patients were treated in Heidelberg and 17 in Pavia. The patients received 28-day cycles of bendamustine (60–100 mg/m2 on days 1 and 2) and prednisone (100 mg on days 1–4). The target dose of bendamustine was 100 mg/m2, and lower doses were used in 20 patients (55%) who had baseline cytopenia. Ten patients (28%) had IgM clones. Of them, 8 received rituximab associated with BeP. Response was evaluated according to the novel criteria of the International Society of Amyloidosis. Patients' characteristics are reported in Table 1. Severe (grade 3 or 4) adverse events (SAE) were observed in 33% of subjects, most common being cytopenia (17%). Other SAEs were fever (6%), portal vein thrombosis, skin rash, renal failure, and weight loss, in 1 patient each. By intention to treat, 17 patients (47%) achieved hematologic response, with complete remission (CR) in 1 case (3%), and very good partial response in 2 (6%). The median time to response was 3 months. The dose of bendamustine administered in each cycle was not associated with response. Three out of 5 treatment-naïve patients responded. Interestingly, amongst the 8 subjects with IgM clones receiving BeP combined with rituximab, 6 (75%) responded (1 CR), including 1 out of 3 subjects who were refractory to previous rituximab. Amongst 10 patients who were refractory to melphalan, bortezomib and lenalidomide, 4 responded to BeP. Cardiac responses were observed in 3 patients (12%), two of whom also had liver response and one improvement of peripheral neuropathy. Overall, 12 patients (33%) died, and 65% of patients are alive after 3 years. Two subjects died within 3 months from treatment initiation due to advanced cardiac amyloidosis. A troponin I concentration >0.1 ng/mL or a high-sensitivity troponin T level >77 ng/L negatively affected survival (median 5 vs. 45 months, P=0.003). In a 3-month landmark analysis, response to BeP conferred a significant survival advantage (Figure 1).
Treatment with bendamustine is effective and well tolerated, representing an additional treatment option in AL amyloidosis, particularly as salvage therapy. Its use in combination with rituximab in IgM patients is very promising, and warrants further studies in prospective international trials.
Patients' characteristics
| Variable . | N (%)/median (range) . |
|---|---|
| Newly diagnosed | 5 (14) |
| Refractory to previous therapy | 24 (67) |
| Relapsed after previous therapy | 7 (19) |
| Number of prior therapies | 2 (0–5) |
| Previous treatment type: | |
| alkylating agents | 29 (81) |
| bortezomib | 16 (44) |
| lenalidomide | 12 (33) |
| thalidomide | 6 (17) |
| rituximab* | 6 (17) |
| Male gender | 18 (50) |
| Age, years | 66 (33–80) |
| Organ involvement | |
| heart | 25 (69) |
| kidney | 20 (56) |
| soft tissues | 13 (36) |
| liver | 10 (28) |
| peripheral nervous system | 8 (22) |
| Two or more organs involved | 22 (61) |
| New York Heart association class III or IV | 14 (39) |
| Cardiac Stage° | |
| I | 9 (28) |
| II | 19 (59) |
| III | 4 (13) |
| Estimated glomerular filtration rate | 22 (61) |
| ≥60 mL/min per 1.73 m2 | 11 (31) |
| 30–59 mL/min per 1.73 m2 | 3 (8) |
| <30 mL/min per 1.73 m2 | |
| Bone marrow infiltration | 15 (3–30) |
| Variable . | N (%)/median (range) . |
|---|---|
| Newly diagnosed | 5 (14) |
| Refractory to previous therapy | 24 (67) |
| Relapsed after previous therapy | 7 (19) |
| Number of prior therapies | 2 (0–5) |
| Previous treatment type: | |
| alkylating agents | 29 (81) |
| bortezomib | 16 (44) |
| lenalidomide | 12 (33) |
| thalidomide | 6 (17) |
| rituximab* | 6 (17) |
| Male gender | 18 (50) |
| Age, years | 66 (33–80) |
| Organ involvement | |
| heart | 25 (69) |
| kidney | 20 (56) |
| soft tissues | 13 (36) |
| liver | 10 (28) |
| peripheral nervous system | 8 (22) |
| Two or more organs involved | 22 (61) |
| New York Heart association class III or IV | 14 (39) |
| Cardiac Stage° | |
| I | 9 (28) |
| II | 19 (59) |
| III | 4 (13) |
| Estimated glomerular filtration rate | 22 (61) |
| ≥60 mL/min per 1.73 m2 | 11 (31) |
| 30–59 mL/min per 1.73 m2 | 3 (8) |
| <30 mL/min per 1.73 m2 | |
| Bone marrow infiltration | 15 (3–30) |
Used in patients with IgM clones.
°Available in 32 patients. Cardiac staging was based on N-terminal pro-natriuretic peptide type-B (cutoff 332 ng/L) and cardiac troponin I (cutoff 0.1 ng/mL) or high-sensitivity troponin T (cutoff 77 ng/L). Stage I patients had both markers below the cutoffs, stage II only one marker above the cutoffs and stage III patients both markers above the cutoffs.
Survival according to response to therapy
Off Label Use: Use of bendamustine in AL amyloidosis.
Author notes
Asterisk with author names denotes non-ASH members.