Abstract
Abstract 418
Diffuse large B-cell lymphoma (DLBCL) is the most common non-hodgkin lymphoma. It can be subdivided into two major subtypes with different gene expression profiles. The first, called germinal center B cell-like (GCB), develops from normal germinal center (GC) B-cells. The second, called Activated B cell-like (ABC), is thought to develop from plasmablasts, a population of B cells at a later stage of differentiation. In agreement with these cellular origins, the somatic hypermutation process (SHM), responsible for an aberrant accumulation of chromosomal translocations and somatic mutations at multiple oncogenes in these pathologies, remains ongoing in all GCB but in only rare ABC cases.
Whether this ongoing SHM activity also participates in clinical evolutions is not known. To address this question, we have reconstructed the phylogenetic trees of 14 refractory/relapsed DLBCL cases by analysing the somatic mutations patterns at the IGH variable regions from different biopsies, obtained at diagnosis and during progressions. These lymphomas were classified as belonging to the GCB or ABC subtype by hierarchical clustering, using the expression of 18 genes that discriminate these two molecular subtypes. For each of the 30 biopsies we analysed, the clonotypic VDJ rearrangement was amplified by PCR, subcloned into the pGEM-T vector, and 10 individual colonies were sequenced. Phylogenetic trees rooted on the closest IGVH germline gene were reconstructed using the SeaView software and the PhyML algorithm. To avoid PCR artefact, only those mutations observed in at least two subclones were considered.
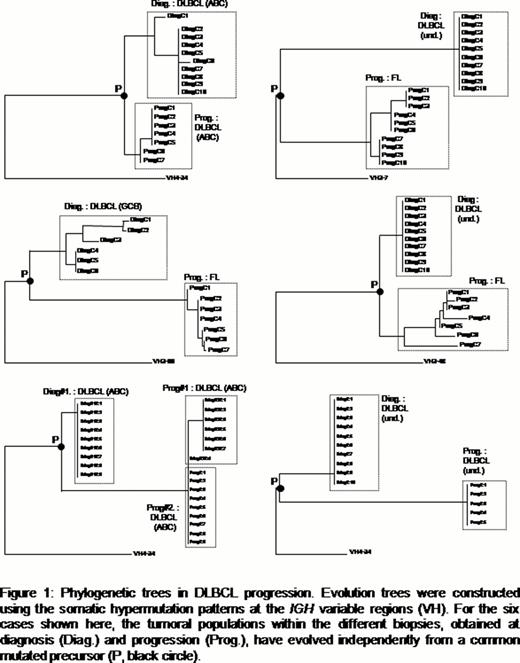
In this series, hierarchical clustering identified 5 ABC and 5 GCB DLBCLs. The four remaining cases showed intermediate gene expression profiles. As expected, intraclonal variations (ICV) were found in all 5 GCB and in only 1 ABC tumor at diagnosis. Three lymphomas, all from the ABC subtype, did not show any evidence of clonal evolution, presenting homogeneous and identical SHM patterns at diagnosis and progression. For five other cases (4 GCB and 1 undefined DLBCL), the evolution trees revealed that the progressions have resulted from the selection of cells already present at diagnosis. Surprisingly, 3 of these 4 GCB cases showed ICV in the first but not in the second biopsy, suggesting that the SHM process was not anymore active in the selected population. Finally, for the six remaining cases (2 ABC, 1 GCB and 3 undefined DLBCLs), our data revealed that the tumoral populations at diagnosis and at relapse have evolved in parallel from less mutated precursors (Figure 1). In these lymphomas, the tumoral cells within the different biopsies shared the same CDR3 region, confirming their clonal origin. They also shared several somatic mutations, suggesting that their precursors were of GC or post-GC origin. Interestingly, 3 of these cases relapsed as lower grade follicular lymphomas (FL). One, from the GCB subtype, showed ICV both at diagnosis and at relapse. The two others, one ABC and one undefined case, showed ICV only at relapse, in FL, but not at diagnosis in DLBCL, again suggesting that the activity of the SHM process can be influenced by other factors than the ABC/GCB signature.
In conclusion, our data show that progressions in DLBCL can develop from the dominant tumoral population or from the selection of minor subclones, maybe due to their inherent capacity to resist treatments. Alternatively, a significant percentage of clinical evolutions apparently develop from a pool of less mutated post-GC precursors, involved in an active diversification process driven by the SHM machinery.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.