Abstract
Abstract 4220
HIV-1 based lentiviral vectors have been shown to transduce human hematopoietic stem cells, but transduction efficiency varies among individuals. Innate immune factors, such as TRIM5α and cyclophilin A (CypA), play an important role for restriction of retroviral infection. We previously demonstrated that genetic variations in rhesus TRIM5α affect transduction efficiency with lentiviral vectors for rhesus hematopoietic repopulating cells (ASH 2011), and an inhibitor of CypA, cyclosporine, decreases variation of transduction efficiency for human CD34+ cells (ASGCT 2011). In this study, we sought to evaluate whether the human innate immune factors TRIM5α and CypA could account for the variation in transduction efficiency for human CD34+ cells with HIV-1 based lentiviral vectors.
Five human T-cell derived cell lines (CEM, CEMx174, A-3, D1.1, and E6-1) were transduced with a GFP-expressing HIV-1 based lentiviral vector at MOI=0.2, 0.5, and 1.0. RNA levels of TRIM5α and CypA at the time of transduction were evaluated by real time PCR, and transduction efficiency (%GFP) was evaluated by flow cytometry 3 days after transduction. Additionally, cell proliferation was evaluated by fold expansion of cell counts during 3 day culture.
All T-cell cell lines revealed an increase of %GFP by MOI escalation, while large variations of %GFP were observed among cell lines (12.5–50.4% at MOI=0.2, 21.3–69.4% at MOI=0.5, and 34.9–84.4% at MOI=1.0). TRIM5α expression levels revealed large differences among all cell lines, while CypA expression levels were similar (815 fold magnitude for TRIM5α, 1.65 fold magnitude for CypA). In all cell lines, %GFP was positively correlated with fold expansion of cell counts (R2=0.87, p<0.05 at MOI=0.2, R2=0.93, p<0.01 at MOI=0.5, and R2=0.95, p<0.01 at MOI=1.0), while %GFP was negatively correlated with relative TRIM5α mRNA levels (R2=0.59, p=0.13 at MOI=0.2, R2=0.72, p=0.07 at MOI=0.5, and R2=0.83, p<0.05 at MOI=1.0). No correlation was observed between %GFP and CypA mRNA levels (R2=0.01, p=0.47 at MOI=0.2, R2=0.10, p=0.52 at MOI=0.5, and R2=0.13, p=0.57 at MOI=1.0).
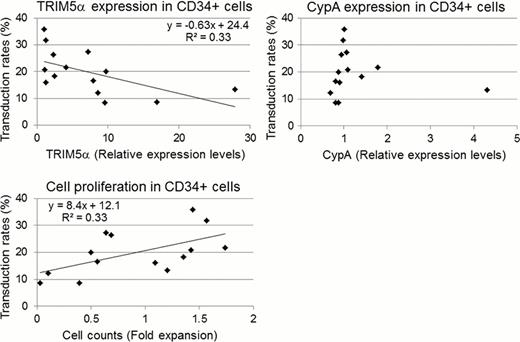
Next, human CD34+ cells from 14 individual donors were transduced with the HIV-1 vector at MOI=50 following a one day prestimulation. As before, RNA levels of TRIM5α and CypA were evaluated at the time of transduction, and %GFP and cell counts were evaluated 3 days after transduction. A large variation of %GFP was observed among individual donors (8.5–35.8%). TRIM5α expression levels also showed a large variation among individual donors, while CypA had similar expression levels in human CD34+ cells (27.7 fold magnitude for TRIM5α, 6.27 fold magnitude for CypA). In donor cells, %GFP was positively correlated with fold expansion of cell counts (R2=0.33, p<0.05) while a negative correlation was seen between %GFP and TRIM5α mRNA levels (R2=0.33, p<0.05) (figure). Again, no correlation was observed between %GFP and CypA mRNA levels (R2=0.02, p=0.65) (figure). Additionally, we sequenced known CypA polymorphisms in coding and promoter regions. Although two polymorphisms in the promoter region were detected (C1868G and A1914G), there was no correlation among %GFP, CypA mRNA levels, and polymorphisms.
In conclusion, human TRIM5α mRNA levels were shown to negatively correlate with transduction efficiency of an HIV-1 based lentiviral vector in both human T-cell cell lines and CD34+ cells, while human CypA mRNA levels and CypA polymorphisms do not correlate with transduction efficiency. Additionally, cell proliferation was positively correlated with transduction efficiency. Our findings are important for both understanding and mitigating the variability of transduction efficiency for human CD34+ cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.