Abstract
Abstract 4260
Patients with chronic-phase, Philadelphia chromosome-positive chronic myeloid leukemia (CML) receiving tyrosine kinase inhibitor (TKI) therapy experience symptoms. We investigated the association between patient-reported symptom severity and longitudinal levels of interference with daily functioning.
In this descriptive, longitudinal study, 156 patients with CML rated the 20 symptom items (13 core cancer and 7 CML-specific symptom items) and 6 interference items of the M. D. Anderson Symptom Inventory for CML (MDASI-CML), on a 0-to-10 scale (0 = not present or no interference; 10 = as bad as can be imagined or complete interference) every 2 weeks for 1 year. Group-based trajectory analysis was used to describe the longitudinal patient-reported interference of symptoms with daily functioning. We used univariate mixed modeling to describe the relationship between symptom severity at time of study entry and patient-reported functional interference across time. These analyses were adjusted for patient factors (age, sex, race, marital status, employment, and time since diagnosis).
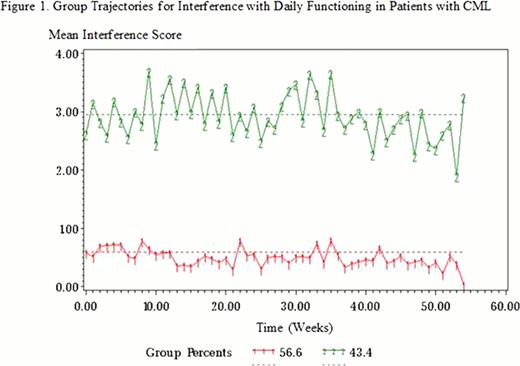
Mean participant age was 51.1 years (standard deviation [sd]=13.5) and mean years of education was 14.6 (sd=2.3); 72 participants (46%) were male; 116 (74%) were white non-Hispanic with 13 (8%) black non-Hispanic, 15 (9%) Hispanic, and 12 (8%) other races; 124 (80%) were married or lived with another adult; 92 (59%) were employed full or part time, 15 (10%) were homemakers, 26 (17%) were retired, 17 (11%) were on a medical leave of absence or medically disabled, and 6 (4%) were unemployed. Participants were mostly in the chronic phase of CML (95.5%), were receiving TKI therapy (98%), had been diagnosed for a mean of 6.1 years (sd=4.74), and been on TKI therapy for a mean of 4.1 years (sd=3.13) before study entry. Most participants had little evidence of disease with 123 (79%) having a complete cytogenetic response and 81 (52%) having a complete molecular response by polymerase chain reaction at time of study entry. Fatigue (mean=2.93, sd=2.67) was persistently the most severe symptom, followed by disturbed sleep (mean=2.19, sd=2.59), drowsiness (mean=2.07, sd=2.40), muscle cramping (mean=2.02, sd=2.46), and difficulty remembering (mean=1.81, sd=2.17). The highest interference reported was with work (mean=1.69, sd=2.41), followed by general activity (mean=1.67, sd=2.32) and mood (mean=1.50, sd=2.15). Trajectory analysis showed that 69 (43%) participants were in a higher interference group (mean interference=3.01, sd=1.60) and that the remaining 87 (54%) were in a distinctly lower interference group (mean=0.50, sd=0.44) (Figure 1). None of the demographic characteristics or time since diagnosis predicted patient interference scores. Eleven symptom scores at study entry significantly predicted interference scores (pain, p=0.0009; fatigue, p<0.0001; disturbed sleep, p< 0.0001; distress, p<0.0001; shortness of breath, p=0.0005; drowsiness, p=0.0003; dry mouth, p=0.0021; sadness, p=0.0092; peripheral edema, p=0.0049; muscle cramping, p=0.0099; malaise, p<0.0001). Participants with higher symptom severity at study entry had higher interference scores across time.
Patients with CML, regardless of disease status, may still experience symptoms that interfere with daily functioning while on oral TKI therapy. Symptoms and their interference with daily functioning can be easily documented by routine assessment and deserve consideration in long-term treatment planning for these patients, as they may interfere with patient compliance with TKI therapy and affect quality of life for survivors with CML.
Williams:Novartis Pharmaceuticals: Research Funding. Ault:Bristol Myers Squibb: Speakers Bureau. Cortes:Bristol Myers Squibb: Consultancy, Research Funding; Novartis Pharmceuticals: Consultancy, Research Funding; Ariad: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.