Abstract
Abstract 4325
Acute myeloid leukemia (AML) is a malignant hematopoietic neoplasm characterized by clonal proliferation of tumor cells that arise from the hematopoietic stem/progenitor population within the bone marrow. Nitric oxide (NO), a signaling molecule regulates hematopoietic stem and progenitor cells maturation by altering cell-cell adhesions, lineage-specific growth factors and actin confirmation (Michurina, et al, 2004). Moreover, suppression of NOS activity has profound effect on hematopoietic stem cells/progenitor cells, by increasing their self renewal or survival potential. Serum nitrite/nitrate level and expression of NOS is found to be more in AML patients (Brabdao et al, 2001). NO was also found to have cytotoxic potential in myeloid leukemia cells (Tsumori et al, 2002). Previous study from this lab has demonstrated that NO donor DETA NONOate in lower concentrations enhanced proliferation of HL-60 cells, while higher concentrations induced cytostasis, mitochondrial membrane potential loss and apoptosis (Kumar et al., 2010).
The present study was undertaken to assess the circulating levels of NO metabolite, nitrite and NOS expressions in the circulating neutrophils and their precursor cells so as to assess the role of NO/NOS in AML pathology.
Blood and bone marrow was collected from AML patients (mean age 33+10 years) after their informed consent. French American British criteria were used to diagnose AML pateints, which was based on the presence of 20% or more myeloblasts in bone marrow/blood smears. Twenty patients of AML were included and the study was approved by the Institutional ethics committee. Neutrophils (PMNs) and their precursor cells were isolated by Percoll density gradient and purity was confirmed by CD11b, CD15, CD16 labeling and by Giemsa staining. Total nitrite level in plasma and PMNs was assessed by Griess reagent and NOS expression was analysed by Real time RT-PCR.
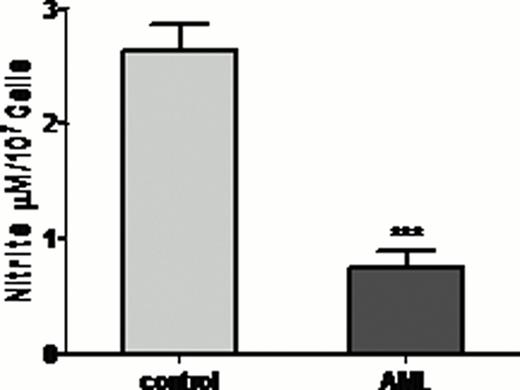
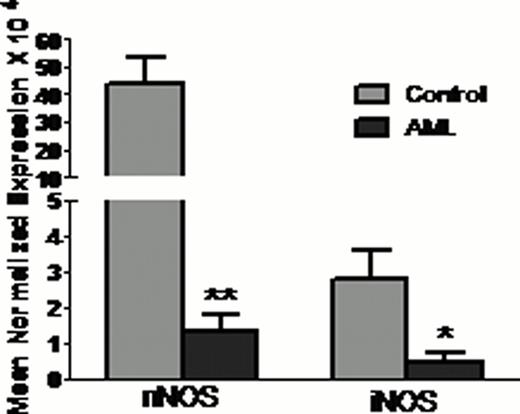
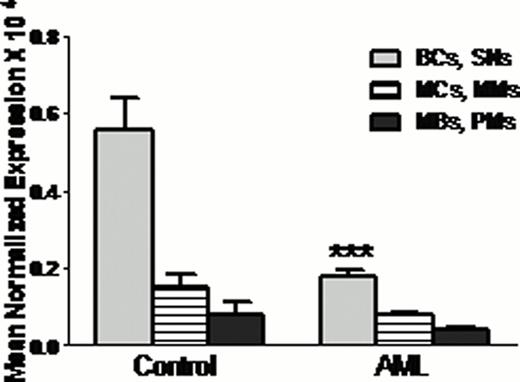
Total nitrite level was less in AML patient's plasma and PMNs compared to healthy control (Figure 1A). Expression of both nNOS and iNOS was also less in AML patient's PMNs (Figure 1B). As AML is a disease of progenitor cells, further we have analysed nitrite level and NOS expression in PMNs progenitor cells {myeloblasts (MBs), promyelocytes (PMs), myelocytes (MCs), metamyelocytes (MMs), band cells (BCs) and segmented neutrophil (SNs)}. PMNs nitrite content and n/iNOS expression was less in all the progenitor cells compared to healthy control progenitor cells (Figure 2). In vitro results also showed that NOS expression was augmented following differentiation of HL60 cells in neutrophils.
These observations indicate that NOS expression was significantly reduced in PMNs of AML patients. Further studies will be undertaken to assess the effect of NO modulators on the cell differentiation and apoptosis in AML patients.
Total nitrite content and NOS expression in PMNs (A) Nitrite content and (B) Expression of nNOS and iNOS in AML patients PMNs. *** p<0.001, ** p<0.01, * p<0.05 vs. Control
Total nitrite content and NOS expression in PMNs (A) Nitrite content and (B) Expression of nNOS and iNOS in AML patients PMNs. *** p<0.001, ** p<0.01, * p<0.05 vs. Control
nNOS expression in neutrophil progenitor cells {myeloblasts (MBs), promyelocytes (PMs), myelocytes (MCs), metamyelocytes (MMs), band cells (BCs) and segmented neutrophil (SNs)}
nNOS expression in neutrophil progenitor cells {myeloblasts (MBs), promyelocytes (PMs), myelocytes (MCs), metamyelocytes (MMs), band cells (BCs) and segmented neutrophil (SNs)}
No relevant conflicts of interest to declare.
References:
Author notes
Asterisk with author names denotes non-ASH members.