Abstract
Abstract 433
Novel gene mutations have been found in CLL by next generation sequencing including mutations of NOTCH1 and SF3B1 in 5–20% of cases. In initial studies, both have been associated with advanced disease and poor outcome.
We assessed the incidence and impact of gene mutations in the CLL8 trial (1st line FC vs. FCR, n=817). TP53 (exons 2–11) was analyzed by a re-sequencing chip (Amplichip, Roche Molecular Systems) with confirmatory Sanger sequencing. NOTCH1 was analyzed by Sanger sequencing exon 34, chr9:139,390,619–139,391,290 (PEST domain). SF3B1 (exons 13–16) was analyzed by DHPLC (WAVE® 3500HT, Transgenomic Inc.) with subsequent Sanger sequencing. Baseline samples were available for analysis of genetic markers in 619 (75.8%) to 645 (78.9%) patients. All markers were available for 573 (70.1%) patients and this cohort was representative of the full trial population.
Mutations (mut) were found in TP53, NOTCH1, and SF3B1 in 11.5%, 10.0%, and 18.4%, respectively. At least one mutation was identified in 35.2% patients, while 30.6% had one, 4.4% had two and 0.2% had three mutations. Concurrent NOTCH1mut and SF3B1mut were found in only 0.5% patients. TP53mut was observed in 16.7% of NOTCH1mut cases (p=.528) and in 14.5% of SF3B1mut patients (p=.472).
Regarding baseline characteristics, there were significant associations of TP53mut with CIRS>1, unmutated IGHV and 17p-; of NOTCH1mut with Binet A/B, no B-symptoms, unmutated IGHV, and 17p-; and of SF3B1mut with TK>10, and no +12.
Regarding response to therapy, TP53mut was significantly associated with refractory disease in both arms (FCR: 25.0% vs. 1.8%, p<.001, FC: 48.4% vs. 7.8%, p<.001,); while NOTCH1mut showed only a trend in the FCR arm (FCR: 10.9% vs. 3.4%, p=.109, FC: 11.9% vs. 12.9%, p=.775); and SF3B1mut did not impact response to therapy (FCR: 3.6% vs. 3.7%, p=1.00, FC: 12.3% vs. 10.9%, p=1.00).
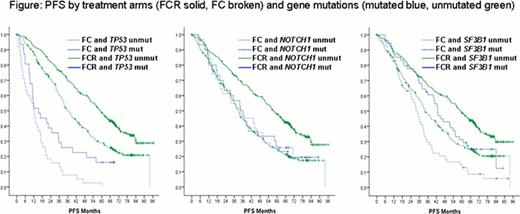
At extended follow-up (median 69.97 months), FCR resulted into significantly improved PFS (HR 0.586, p<.001) and OS (HR 0.678, p=.001). TP53mut was associated in both treatment arms with significantly decreased PFS (FC: HR 4.295, p<.001; FCR: HR 3.173 p<.001) and OS (FC: HR 4.642 p<.001; FCR: HR 4.447, p<.001). In contrast, NOTCH1mut was only in the FCR arm associated with significantly decreased PFS (FC: HR 0.931, p=.741; FCR: HR 1.718, p=.013) and a trend to inferior OS (FC: HR 0.854, p=.605; FCR: HR 1.610, p=.112). SF3B1mut was associated in both treatment arms with significantly decreased PFS (FC: HR 1.520, p=.009; FCR: HR 1.463, p=.033) and a trend to inferior OS (FC: HR 1.338, p=.178; FCR: HR 1.305, p=.301).
To evaluate the independent prognostic impact, we performed multivariable analyses by Cox regression for PFS and OS including the following variables: treatment, age, sex, stage, ECOG status, B-symptoms, WBC, TK, β2-MG, 11q-, +12, 13q-, 17p-, IGHV, TP53, NOTCH1 and SF3B1. Regarding PFS, the following independent prognostic factors were identified: FCR (HR 0.510, p<.001), TK>10 (HR 1.367, p=.019), IGHV<98% (HR 1.727, p<.001), 11q- (HR 1.536, p<.001), 17p- (HR 2.949 p<.001), TP53mut (HR 2.113 p<.001), and SF3B1mut (HR 1.348, p=.024). Regarding OS, the following independent prognostic factors were identified: FCR (HR 0.701, p=.049), ECOG>0 (HR 2.202, p<.001), TK>10 (HR 2.707, p<.001), IGHV<98% (HR 1.547, p=.055), 17p- (HR 3.546 p<.001) and TP53mut (HR 3.032 p<.001).
To identify a predictive impact of gene mutations for a specific treatment effect by the addition of rituximab, we performed multivariable analyses including the treatment arms, the gene mutations and the interaction of both. Regarding PFS, FCR (HR 0.544, p<.001), TP53mut (HR 3.607, p<.001), SF3B1mut (HR 1.355, p=.012) and NOTCH1mut interaction with FCR (HR 1.652, p=.022) were identified as independent factors. Regarding OS, FCR (HR 0.654, p=.002) and TP53mut (HR 4.470, p<.001) were identified as independent factors while NOTCH1mut interaction with FCR (HR 1.331, p=.344) showed a trend. The interaction between NOTCH1mut and FCR treatment is illustrated in univariate PFS analysis, in which the addition of rituximab led to a benefit only among patients without NOTCH1mut (Figure).
In conclusion, gene mutations show independent prognostic value for PFS (TP53, SF3B1) and OS (TP53) in patients receiving 1st line FC and FCR treatment. Of note, NOTCH1mut appears to identify a subset of CLL patients that does not benefit from the addition of rituximab to FC.
Stilgenbauer:Roche: Consultancy, Honoraria, Research Funding. Patten:Roche: Employment. Wenger:Roche: Employment. Mendila:Roche: Employment. Hallek:Roche: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.