Abstract
Abstract 4593
NOX-A12 is a novel, potent, L-aptamer inhibitor of CXCL12/SDF-1, a chemokine which attracts and activates immune- and non-immune cells. The signaling of CXCL12 has been shown to play an important role in the pathophysiology of chronic lymphocytic leukemia (CLL), especially in the interaction of leukemic cells with their tissue microenvironment. The therapeutic concept of NOX-A12 is to inhibit such tumor-supporting pathways and thereby sensitizing the CLL cells towards chemotherapy.
The purpose of this phase IIa study is to evaluate the safety and efficacy of NOX-A12 in combination with background chemo-immunotherapy of bendamustine and rituximab (BR) in patients with relapsed CLL. The described study is being performed in compliance with ethical principles based on the Declaration of Helsinki and ICH-GCP guidelines. The study population was split into a pilot and expansion group. In the pilot group, 3 cohorts of 3 patients each received escalating doses of single agent NOX-A12 two weeks prior to the combined treatment of NOX-A12 and BR. Interim data from these patients are reported. Based on previous Phase I studies in healthy volunteers, pilot patients received a dose of 1, 2 or 4 mg/kg body weight (BW) single agent NOX-A12 on day -14, followed by a 2-weeks period of safety, PK and PD assessments prior to the combined treatment with NOX-A12 and BR. To date, the first cohort of the pilot group already progressed to the 2nd cycle of combined treatment. Evaluation criteria included adverse events according to CTCAE V4, flow cytometry of peripheral blood CD34+ cells and CLL cells, pharmacokinetics of NOX-A12, plasma concentration of CXCL12 and tumor response (NCI-WG 1996 criteria, updated 2008).
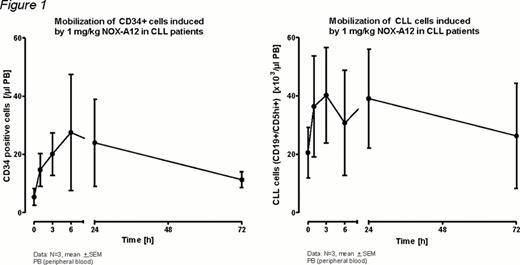
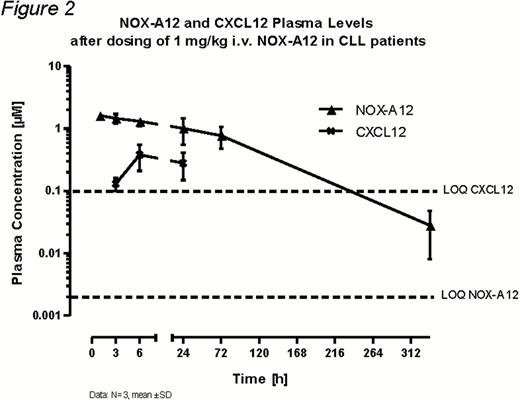
To date 3 patients (age range: 58 – 65 years) have been enrolled in the pilot group of this study. They had received 1 or 2 prior therapies, but no bendamustine. Single i.v. doses of 1 mg/kg BW NOX-A12 had no clinically relevant effects on vital signs, 12-lead ECG parameters and laboratory parameters. One patient reported grade 1 pain in the lower limbs two days after treatment with NOX-A12. This event was not dose-limiting and resolved spontaneously on the same day. Flow cytometry of CD34+ cells and CLL cells (CD19+/CD5+high) showed a rapid mobilization of these cells into the peripheral blood on day 1. Interestingly, return to baseline was not complete at the last assessment on day 3 (for details see Figure 1). The NOX-A12 pharmacokinetics in these 3 patients (for concentration-time profile see Figure 2) is very comparable to healthy volunteers receiving i.v. NOX-A12, with a maximum plasma concentration of 1.52 ± 0.14 μM after 1 h (tmax) and a plasma elimination half-life of about 50 h. As seen in healthy volunteers the plasma concentration of CXCL12 increased upon NOX-A12 treatment and reached a maximum of 0.434 ± 0.076 μM at 24 to 72 h p.a. without ever approaching the plasma concentration of NOX-A12 (Figure 2).
Single i.v. doses of NOX-A12 at 1 mg/kg BW were safe and well tolerated; the maximum tolerated dose was not reached. NOX-A12 induced a long-lasting mobilization of CD34+ cells and leukemic cells in patients with relapsed CLL, consistent with a mechanism of action based on CXCL12 inhibition. Patient accrual and identification of an optimal chemosensitization regimen of NOX-A12 combined with BR is being continued.
Vauléon:NOXXON Pharma AG: Employment. Zöllner:NOXXON Pharma AG: Employment. Dümmler:NOXXON Pharma AG: Employment. Kruschinski:NOXXON Pharma AG: Employment. Fliegert:NOXXON Pharma AG: Employment.
Author notes
Asterisk with author names denotes non-ASH members.