Abstract
Abstract 490
Atypical hemolytic uremic syndrome (aHUS) is a clinically defined thrombotic microangiopathic anemia (TMA) characterized by the symptom triad of renal failure, thrombocytopenia and microangiopathic hemolytic anemia in the absence of Shiga-toxin-producing bacteria as a triggering factor. Its pathogenesis is linked to dysregulation of the alternative pathway of the complement cascade, with loss-of-function mutations reported in complement regulators like factor H (CFH), membrane cofactor protein (MCP), factor I (CHI) and thrombomodulin (THBD), and gain-of-function mutations in complement activators like factor B (CFB) and complement component 3 (C3). In nearly 40% of patients, however, mutations are not identified in these genes raising the possibility of unrecognized contributory genetic causes.
Genetic variants in ADAMTS13 (a disintegrin and metalloprotease with thrombospondin motifs) have been causally related to thrombotic thrombocytopenic purpura (TTP), another TMA characterized by the pentad of neurologic symptoms, fever, kidney failure, thrombocytopenia and microangiopathichemolytic anemia. The phenotypic similarities between aHUS and TTP can at times lead to difficulty in their clinical distinction. On this basis, we hypothesized that partial deficiency in ADAMTS13 function may coexist with abnormalities in the alternative complement pathway in patients with aHUS.
To test this hypothesis, we measured ADAMTS13 functional activity in 26 patients with aHUS by fluorescence resonance energy transfer substrate-von Willebrandfactor 73mer (FRETS-VWF73) and recombinant VWF A2 domain cleavage assay. We also genotyped all patients for ADAMTS13 and alternative complement pathway genes. We transiently expressed 10 different ADAMTS13 missense variants in HEK293 cells to analyze activity and secretion of each recombinant protein.
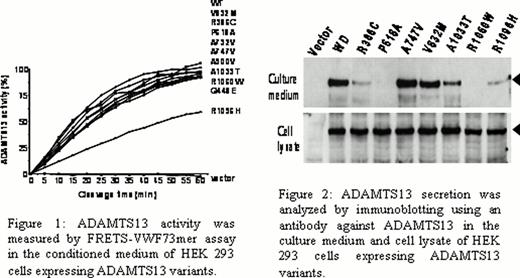
The ADAMTS13 functional activity was partially reduced (< 60%) in serum from 20 patients (77%) as measured by FRETS-VWF73 and recombinant VWF A2 cleavage assays. Genetic variants in ADAMTS13 were identified in 21 patients (81%) and included R386C, Q448E, A900V, V832M, R1060W, R7W/Q448E, A900V/Q448E, R1096H/A747V, R7W/A1033T, R7W/P618A/Q448E, R7W/P618A/A900V/Q448E, R7W/P618A/A732V/Q448E. The coexistence of both ADAMTS13 deficiency and excessive complement functional activity was present in 13 patients (50%). Activity of recombinant ADAMTS13 proteins containing the following variants was normal: R386C, Q448E, P618A, A732V, A747V, V832M, A900V, A1033T and R1060H. The R1096H variant of ADAMTS13 was associated with a reduction in functional activity by 50%. The secretion of recombinant proteins with the following variants was severely reduced: P618A, R1060H (<1%), R386C, R1096H (<10%), and A1033T (<50%) (Figures 1 and 2).
This study implicates ADAMTS13 in the pathogenesis of aHUS and mandates the evaluation of ADAMTS13 in aHUS patients. Defining the role of ADAMTS13 in aHUS may aid in the development of new treatments and/or assist in clarifying whether long-term treatment with currently available anti-complement therapy is required for this disease.
Kroll:Aplagon: Membership on an entity's Board of Directors or advisory committees; Optimer: Consultancy; Leo: Honoraria, Travel Expenses, Travel Expenses Other.
Author notes
Asterisk with author names denotes non-ASH members.