Abstract
Abstract 4938
The effect of azacitidine (AZA) on health related quality of life (QOL) compared with best supportive care in MDS patients has been evaluated by Kornblith A. et al (JCO 2002) in a prospective randomized CALGB trial. The study demonstrated improved fatigue, dyspnea, physical functioning and psychological state in patients receiving AZA. One limitation is the relatively short follow up (last QOL measured at 8. 6 months) and the lack of validation in a non-trial setting. We have been conducting prospective assessments of QOL in all patients registered at our MDS clinic using the instruments EORTC QLQ-C30, FACT-Fatigue, EQ-5D and a global fatigue scale. We present longitudinal data on 56 registered patients registered in our program who were treated with AZA, 50 with serial QOLs.
We examined and compared QOL scores at AZA start (baseline) and over time in all patients. We considered the following co-variates' potential impact on QOL scores: age, sex, IPSS, time from diagnosis, being a responder, # cycles and transfusion dependence. We used univariate linear regression analysis for continuous variables and analysis of variance (ANOVA) for categorical variables to determine their relationship with QOL scores at baseline and over time. For time-dependent covariates, linear mixed model was performed with random intercept and unstructured covariance matrix. Clinically significant (CS) score differences were considered 10 points for the QLQ-C30, 4 for the FACT-Fatigue and 0. 08 for the EQ-5D (7 for the visual analog score). The impact of baseline covariates on the QOL scores for global health, fatigue and dyspnea were determined by backward selection procedure of regression analysis using the Bonferroni adjusted P value of <0. 01 for multiple comparisons. Patients provided informed consent for this REB approved study.
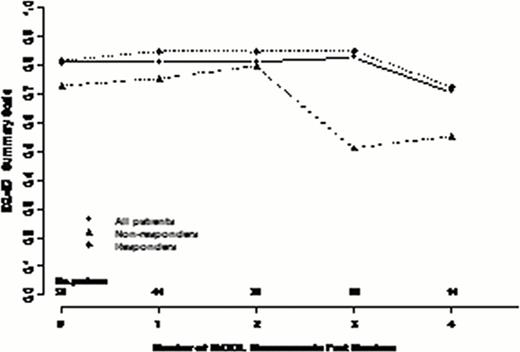
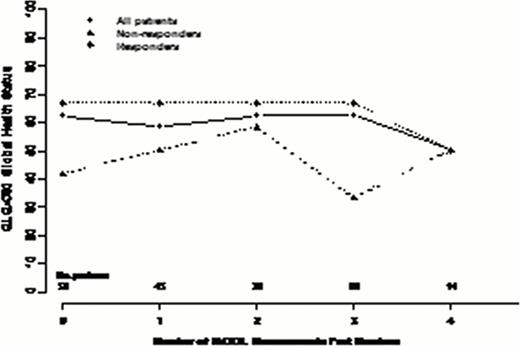
56 MDS patients consented to our registry have been treated with AZA between Oct 2008 and July 2012. The median age was 72, 60% were male and 77% had int-2/high risk IPSS MDS. 64 % were transfusion dependent (TD) at baseline. With a median time to death or last follow up of 16 months (range 1. 5–45) a median of 11 cycles of AZA were administered with 34% remaining on drug for a median of 25 cycles (IQR 8–32). The overall response rate (ORR) was 62%: 25% CR; 5% MCR; 4% PR; 28% HI. Stable disease (SD) was seen in 26% and not considered a response. 53% became transfusion independent (TI). 59% have died and 61% developed leukemia or progressed to > 30% blasts at a median time of 13 months. Overall survival was 18 months (95% CI 14. 5–26). 50 were evaluable for HrQOL with a median time between each serial QOL of 13 weeks (IQR 10–18). QOL was assessed at baseline (within 12 weeks pre-AZA start) in 50 patients, 2x in 44, 3x in 32, 4x in 22 and 5x in 14 patients (exceeding 52 weeks follow up). Looking at all 50 patients, overall, function and symptom domains remained stable over time in all instruments. At baseline, there were no statistically significant differences in QOL scores between responders and non responders. Nevertheless, clinically important differences were seen in physical, role, cognitive and social functioning, global health status (all higher in responders). Assessing QOL changes over time and considering baseline and time-dependent predictive factors in multivariate analysis, responders had significantly superior global health status (p=. 001) and EQ-5D scores (p=. 0002) and lower levels of fatigue (p<. 0001). If transfusion dependence status at time of QOL was included in the model, this often supplanted response as predictive of higher scores over time, likely representing the strong relationship between response and transfusion dependence.
In addition to validating the clinical outcomes of AZA-001 study, we validate the importance of clinical response on QOL in MDS patients treated with AZA. Unlike the CALGB study, we observed relative stability in global health status and fatigue scores in responding patients and overall declines in non-responding patients. The higher scores at baseline in the patients destined for response raises the intriguing possibility that QOL at AZA start may be independently predictive of response perhaps because of improved tolerability and ability to remain on drug for the requisite number of cycles to achieve response.
Changes in select QOL scores over repeated measures in all patients and according to response
Changes in select QOL scores over repeated measures in all patients and according to response
Wells:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Buckstein:Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.