Abstract
Abstract 500
Rivaroxaban for Thromboprophylaxis after Total Hip or Knee Replacement Surgery: Comparison of Outcomes of the XAMOS and RECORD Studies.
Patients undergoing major orthopedic surgery are at risk of venous thromboembolism (VTE). Rivaroxaban has been approved for clinical use in this indication based on the extensive phase III RECORD program, which investigated the efficacy and safety of oral rivaroxaban regimens compared with subcutaneous enoxaparin regimens in patients undergoing elective total hip or knee replacement surgery. XAMOS is a phase IV, non-interventional, open-label cohort study that compared rivaroxaban with any pharmacological prophylaxis used in routine clinical practice for VTE prevention after major orthopedic surgery of the hip or knee. An additional aim of the XAMOS study was to assess whether the results from the phase III RECORD studies would be reflected in routine clinical practice.
The XAMOS study, which ran from 2009 to 2011, collected data on the incidence of adverse events (including symptomatic thromboembolic and bleeding events) in adult patients from 252 centers in 37 countries worldwide, who underwent elective hip or knee replacement surgery (or hip fracture surgery, where appropriate) and received rivaroxaban or other pharmacological prophylaxis (standard of care). The attending physician determined the type, duration, and dose of drug. Of the 17,701 patients enrolled, 8778 received rivaroxaban and 8635 received standard of care, of whom 7055 (81.7%) received low molecular weight heparin (LMWH). Primary hip/knee replacement surgery accounted for >90% of all procedures. Baseline demographics (e.g. age, sex, body weight, and ethnicity) were similar for patients receiving rivaroxaban, standard of care, or LMWH, and were also similar to those of patients who received rivaroxaban in the RECORD program.
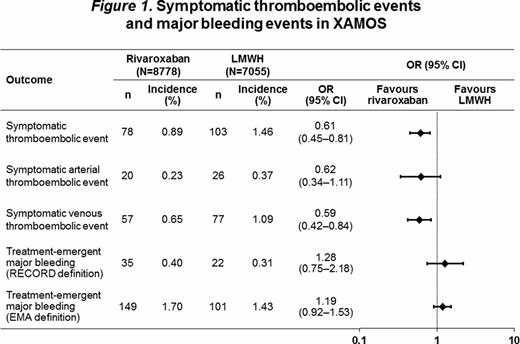
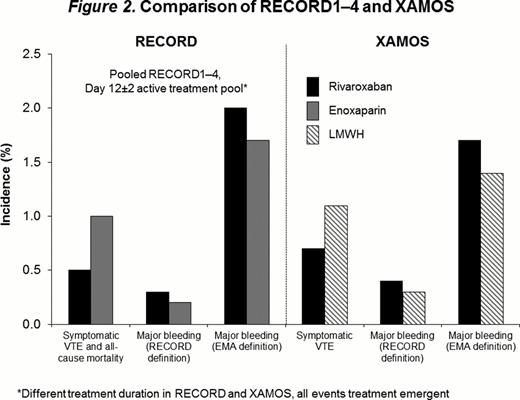
LMWH was the comparator in the RECORD program; therefore, the XAMOS study also compared outcomes with LMWH and rivaroxaban. Data for the LMWH group were similar to the standard-of-care group. Rates of symptomatic arterial, venous, and all thromboembolic events in XAMOS were lower in the rivaroxaban group compared with the LMWH group (Figure 1). The data are consistent with the results of the RECORD1–4 pooled analysis, which showed a significantly lower incidence of symptomatic VTE and all-cause mortality in patients receiving rivaroxaban compared with those receiving enoxaparin (Figure 2). The rates of treatment-emergent major bleeding events (RECORD definition) were 0.4% vs 0.3% in the rivaroxaban and LMWH groups, respectively (odds ratio [OR]=1.28; 95% confidence interval [CI] 0.75–2.18; Figures 1 and 2), and were similar to those in the RECORD1–4 pooled analysis (day 12±2 active treatment pool: 0.3% [rivaroxaban] vs 0.2% [enoxaparin]; OR=1.62; 95% CI 0.77–3.53; Figure 2). When the European Medicines Agency (EMA) definition for treatment-emergent major bleeding was used, the incidence of major bleeding was slightly higher (nonsignificant) for patients receiving rivaroxaban compared with those receiving LMWH in the XAMOS study (1.7% vs 1.4%; OR=1.19; 95% CI: 0.92–1.53; Figures 1 and 2); these results were also similar to those of the RECORD1–4 pooled analysis (day 12±2 active treatment pool: 2.0% [rivaroxaban] vs 1.7% [enoxaparin]; OR=1.20; 95% CI: 0.91–1.57).
XAMOS is the first study presenting real-world experience of the efficacy and safety of rivaroxaban for the prevention of VTE after major orthopedic surgery of the hip or knee. The results of XAMOS showed that rivaroxaban was associated with a significantly lower incidence of symptomatic VTE compared with LMWH, without a significant increase in the risk of major bleeding. These findings are consistent with those obtained in the RECORD studies, and indicate that the results of the RECORD program can be translated into the routine clinical setting.
Turpie:GSK: Speakers Bureau; GSK, JnJ: Consultancy. Schmidt:Bayer: Employment, Equity Ownership. Lassen:Bayer: Speakers Bureau; Bristol-Myers Squibb; GlaxoSmithKline; Bayer; Boehringer: Consultancy; Sanofi-Aventis: Research Funding. Mantovani:Bayer Healthcare Pfizer Merck Serono Baxter Astellas: Speakers Bureau; Bayer Healthcare Pfizer Bristol-Meyer Squibb Amgen Merck&Co: Consultancy; Bayer Healthcare Pfizer Bristol-Meyer Squibb Biogen Idec Medtronic Admirall Otsuka Amgen Merck&Co Merck-Serono: Research Funding. Kreutz:Bristol-Myers Squibb, Bayerm Daichii Sankyo, Berlin-Chemie Menarini: Speakers Bureau; Bayer, Daichii Sankyo: financial and material support, financial and material support Other. Holberg:Bayer Healthcare: Employment. Haas:Sanofi Aventis: Speakers Bureau; Sanofi Aventis, Bristol-Myers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.