Abstract
Abstract 503
The optimal dosing strategy of low molecular weight heparins (LMWH) for the treatment of antenatal venous thromboembolism (VTE) is not known. The physiological changes associated with pregnancy alter the pharmacokinetic (PK) profile of LMWH, and this has led to controversy and subsequent variation in practice, when pregnant women are managed with LMWH for VTE [Voke et al., 2009]. The perceived impact of these PK changes has led to the Royal College of Obstetrics and Gynaecologists (RCOG) recommending a twice daily dose of LMWH, whilst the American College of Chest Physicians recommend that either a once or twice daily dose of LMWH can be used in this setting. We conducted a population PK modelling study of enoxaparin during pregnancy in order to address this issue. Women requiring antenatal enoxaparin, all indications and doses, referred to the specialist haematology clinic at King's College Hospital, a London teaching hospital, were eligible to enrol in the study. Recruited women were reviewed monthly in clinic during their pregnancy, and had up to three anti-Xa activities (trough, 1 hour post dose, and 3 hours post dose) drawn at each clinic attendance. This anti-Xa sampling strategy was based on d-optimal design, to ensure gestation related changes in enoxaparin clearance (CL) and volume of distribution (Vd) were captured in the PK model developed [van Hasselt et al., 2012]. Women under 18 years of age, those with impaired renal function and those non-adherent to enoxaparin were excluded. Compartmental PK modelling was conducted using non linear mixed effects modelling (NONMEM version 7.2.2). One hundred and twenty three patients contributed 795 anti-Xa activities (712 antenatal, 83 postnatal), for PK modelling purposes. A one compartment model with exponential between subject variability on CL and Vd, with a combined proportional and additive error model best fitted the data, with both enoxaparin CL and Vd found to have increased during pregnancy (table).
| Enoxaparin PK parameter . | CL (L/hr) . | Vd (L) . |
|---|---|---|
| Non-pregnancy (literature values) | 0.5-0.7 | 3-7 |
| Pregnancy (this study) | 1.6 | 13.2 |
| Enoxaparin PK parameter . | CL (L/hr) . | Vd (L) . |
|---|---|---|
| Non-pregnancy (literature values) | 0.5-0.7 | 3-7 |
| Pregnancy (this study) | 1.6 | 13.2 |
Goodness of fit plots, a bootstrap analysis and a visual predictive check revealed that a robust enoxaparin model for use in pregnancy had been developed.
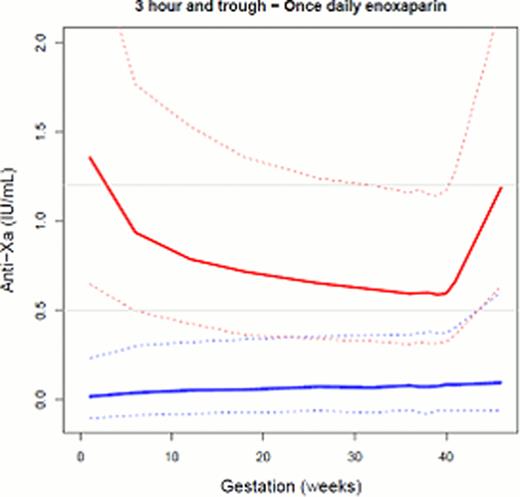
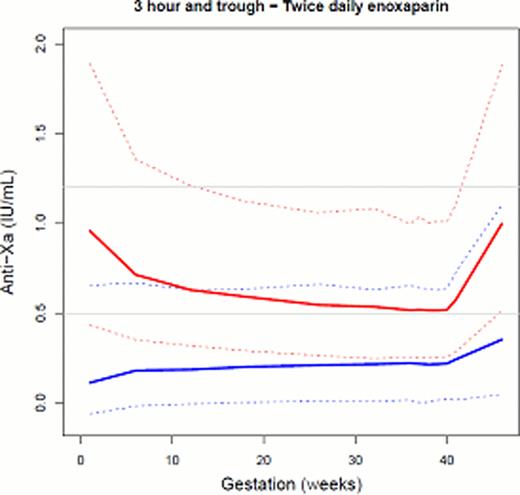
Simulations of women injecting enoxaparin once versus twice daily demonstrate that both dosing regimens will reach RCOG target 3 hour plasma concentrations throughout the duration of the pregnancy (Figure 1). This should provide confidence to clinicians who prescribe a once daily dose of enoxaparin for the antenatal population. Perhaps the more important concentration to assess however is the trough anti-Xa activity, due to the historical thinking that during pregnancy CL increases, therefore so should the dose/dosing frequency. When the trough activity is simulated, the majority of women on a once daily dose, will have measurable anti-Xa activity; with both once and twice daily regimes demonstrating an increase in trough anti-Xa activity with the progression of pregnancy. This occurs due to the increase in Vd and demonstrates that enoxaparin never reaches a steady state during pregnancy. The measurable trough activity confirms the appropriateness of a once daily dose of enoxaparin in this setting.
Our work provides compelling evidence that a once daily dose of enoxaparin for the treatment of antenatal VTE is appropriate. As a result, the current guidelines should be revised.
Simulation of 3 hour (red) and trough (blue) anti-Xa activity of women receiving once daily and twice daily treatment doses of enoxaparin during their pregnancy. The solid line is the median concentration and the dashed lines represent the 5th and the 95th percentile concentrations. Grey horizontal lines represent RCOG 3 hour target anti-Xa concentration.
Simulation of 3 hour (red) and trough (blue) anti-Xa activity of women receiving once daily and twice daily treatment doses of enoxaparin during their pregnancy. The solid line is the median concentration and the dashed lines represent the 5th and the 95th percentile concentrations. Grey horizontal lines represent RCOG 3 hour target anti-Xa concentration.
Off Label Use: Enoxaparin use during the antenatal period.
Author notes
Asterisk with author names denotes non-ASH members.