Abstract
Abstract 5168
Thalassemia is the most common genetic disorder in Egypt composes a major health problem with an estimated carrier rate of 5. 3%-9%. Registered cases in large centers in Egypt September 2007 were 9912 cases, and in Cairo University hematology clinic were alone 2597 cases. Patients with thalassemia major requiring regular blood transfusions accumulate iron that is toxic to the heart, liver, and endocrine systems.
Hepcidin, is a 25 amino-acid peptide hormone synthesized in the liver is a key regulator of iron homeostasis. Recently, hepcidin was reported to bind to the trans-membrane iron exporter “ferroportin” which is present on macrophages, the basolateral site of entrecotes, and in hepatocytes. Hepcidin induces the internalization and degradation of ferroportin. liver hepcidin controls reduction of iron uptake and release. There is also evidence for local production of hepcidin by macrophages, fat cells and cardiomyocytes. Thus, hepcidin is involved in different regulatory mechanisms that control iron imbalance.
The aim of this study is to assess the level of serum hepcidin in hereditary chronic hemolytic anemias and correlate its level to the need for blood transfusion (frequency of blood transfusion) and the serum ferritin level.
Seventy pediatric patients with hereditary chronic hemolytic anemias were the subjects of this study; 53 thalassemia major (TM), 10 thalassemia intermedia (TI), 4 congenital spherocytosis(CS) and 3 sickle cell disease(SCD) patients, mean age 7. 8+3. 9 years (range 1–14). Seventy normal children age and sex matched were studied as controls. Serum hepcidin was measured in all patients and controls by ELISA technique. Serum Hepcidin was measured in all patients one day pre transfusion and in 20 TM patients 3–4 days post transfusion.
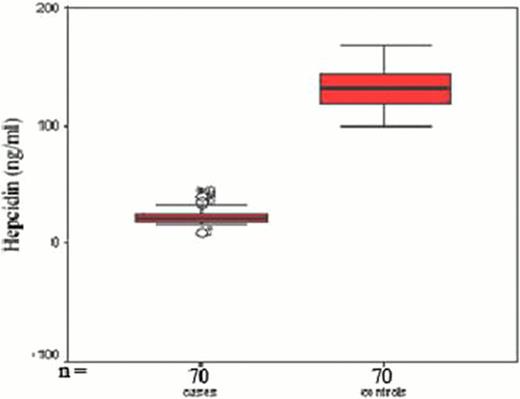
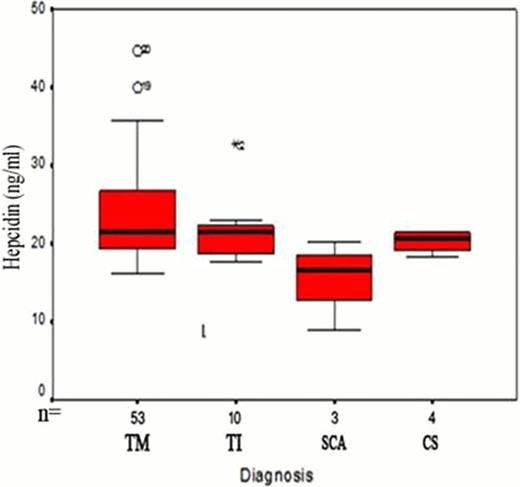
Significant decrease in serum hepcidin levels in all patients compared to controls (mean 22. 9 ±6 vs 132. 4±16. 7 ng/ml, P <0. 001). Hepcidin levels were higher in TM (mean 23. 7±6. 2 ng/ml) than in TI patients (mean =21. 8±4 ng/ml) (Fig 1). The median number of blood transfusions in TM was 70/year (range18–120), in TI the median was 7/year (range 6–17). A 280% increase of serum hepcidin levels of pre transfusion levels was found post transfusion in TM patients (n=20). A significant positive correlation was found between serum hepcidin and frequency of blood transfusion (r=0. 4, p<0. 001), serum ferritin (r=0. 28, p <0. 05) and CRP (r=0. 4, p<0. 05). The hepcidin to ferritin ratio a marker of the hepcidin expression relative to the degree of iron burden was significantly less than one in TM and TI patients (0. 03± 0. 004 and 0. 025± 0. 002 respectively) and far from the level in normal controls (mean 2. 3±0. 7, P<0. 001). Hepcidin and hepcidin/ferritin ratio as markers of iron overload in our patients showed high sensitivity and specificity (99% and 98%, 97% respectively) (table 1, fig 2).
This study examined serum hepcidin and hepcidin/ferritin ratio in hereditary chronic hemolytic anemias, significant low levels were detected. The increase in serum hepcidin level in TM than TI and its marked increase post transfusion in TM patients can be explained by the positive correlation between frequency of blood transfusion and serum hepcidin level in this study. Hepcidin and hepcidin/ferritin ratio can be used as valid markers of iron overload in hereditary chronic hemolytic anemias. Evidence from laboratories around the world have converged on hepcidin as a rational therapeutic agent for treatment of B-thalassemia. Treatment with a hepcidin agonist, at a carefully defined dose, has the potential to ameliorate several aspects of TI due to the specific reduction of iron overload and splenomegaly. Testing this approach provides an exciting opportunity to improve the current management strategies for these diseases, and our study agrees with this approach.
Plots of mean and 95% confidence interval serum hepcidin in cases and control, and in different types of chronic hemolytic anemias.
Plots of mean and 95% confidence interval serum hepcidin in cases and control, and in different types of chronic hemolytic anemias.
Validity of hepcidin (pretransfusion) in case of iron overload
| Hepcidin/ferritin ratio . | Hepcidin . | Variables . |
|---|---|---|
| 0.6 | 70 | Best cut off |
| 0.98 | 0.99 | Area under the curve |
| 99% | 99% | Sensitivity |
| 97% | 98% | Specificity |
| 96% | 99% | PPV |
| 97% | 98% | NPV |
| Hepcidin/ferritin ratio . | Hepcidin . | Variables . |
|---|---|---|
| 0.6 | 70 | Best cut off |
| 0.98 | 0.99 | Area under the curve |
| 99% | 99% | Sensitivity |
| 97% | 98% | Specificity |
| 96% | 99% | PPV |
| 97% | 98% | NPV |
Table (1) and Fig (2) show that both hepcidin and hepcidin/ferritin ratio are valid markers in hereditary chronic hemolytic anemias.
Table (1) and Fig (2) show that both hepcidin and hepcidin/ferritin ratio are valid markers in hereditary chronic hemolytic anemias.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.