Abstract
Abstract 546
TP53 is commonly mutated across lymphoma entities. TP53 mutations have been found in 20–25 % of aggressive B-cell lymphomas and most studies suggest an impact on outcome. Few studies have addressed the impact of TP53 mutations in larger trial cohorts. In order to evaluate the contribution of TP53 mutation status to current risk models, we investigated TP53 gene mutations within the RICOVER-60 trial of the DSHNHL.
Of 1222 elderly patients (aged 61–80 years) randomly assigned to six or eight cycles of CHOP-14 with or without Rituximab, 265 patients (representative of the whole cohort) were analyzed for TP53 mutations. Genomic DNA samples extracted from paraffin embedded tissue were used and mutations were studied by Sanger sequencing of exons 4–9. All patients had untreated CD20+ aggressive B-cell lymphoma according to the World Health Organization classification as confirmed by reference pathology. The primary endpoint of the trial was event-free survival. Analyses were done by intention to treat. The trial is registered on National Cancer Institute website (NCT00052936).
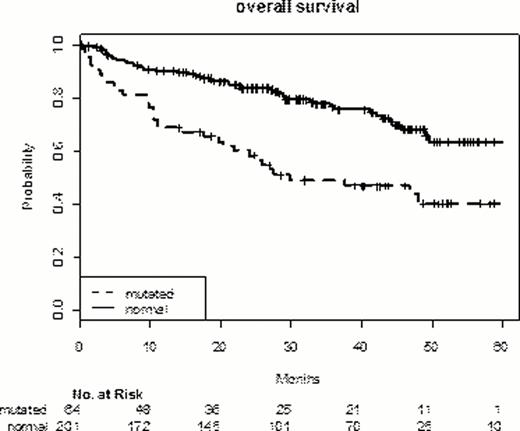
TP53 mutations were demonstrated in 64 of 265 patients (24.1%). The incidence is in accordance to prior studies. TP53 mutation was associated with higher LDH (66% vs. 37%), higher IPI-Scores (IPI 4/5 27% vs. 12%), and B-symptoms (41% vs. 24%) (TP53 mutant (mut) and wild type (wt) groups resp.). Patients with TP53 mutation were less likely to obtain a CR/Cru 62.5% (mut.) vs. 79.6% (wt). The median observation time was 40.2 months and the presence of TP53 mutations was associated with decreased OS; EFS and PFS. The respective 3 year overall and event-free survival were 49% (vs. 77%) and 41% (vs. 60.5%) for the group with TP53 mutation (p<0.0001; <0.01).
The analysis of the TP53 mutant subgroup (n=64) showed no impact of the treatment arm (R-CHOP vs. CHOP; 6 vs. 8 cycles) for OS, EFS or PFS in univariate analysis.
In a Cox proportional hazard models including the IPI, ECOG, LDH, stage and treatment arms, TP53 mutation was shown to be an independent predictor of EFS (HR 1.5), PFS (HR 2.1) and most importantly OS (HR 2.4; p<0.001). Additional factors associated with EFS were LDH, ECOG, stage and Rituximab arm.
Our findings suggest that TP53 mutations are independent predictors of poor PFS, EFS and OS in untreated patients with aggressive CD20+ lymphoma. Based on the analysis within a homogenous trial cohort, strategies to improve outcome for the 20–25% of patients with mutant p53 should be developed. Recurrent genetic lesions should be integrated into risk models in DLBCL.
Overall survival and TP53 mutation in aggressive B-NHL
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.