Abstract
Abstract 583
Granulocyte colony-stimulating factor (G-CSF) is the most commonly used drug for stem cell mobilization. Unfortunately, 5–30% of patients fail to collect sufficient hematopoietic stem/progenitor cells (HSPCs) necessary for transplant. New strategies are needed to increase HSPCs collection in these patients. Apart from cytotoxic effect, bortezomib decreases expression of cell adhesion molecules including VCAM-1. Therefore, we hypothesize that bortezomib can mobilize HSPCs.
C57BL/6 (B6) mice were injected with intravenous (IV) bortezomib (0.8 mg/kg) or Phosphate buffered saline (PBS). Blood were harvested at baseline and 12, 15, 18, 21, and 24 hours (h) after injections and plated on MethoCult media (StemCell Technologies). For evaluation of mechanism and source of mobilized HSPCs, bortezomib versus PBS experiments were performed in splenectomized B6 mice and VLA4 knockout (VLA4KO) mice in addition to B6 mice. Experiments involving combination of bortezomib with G-CSF, AMD3100, and Cytoxan were also conducted in B6 mice. In bortezomib-G-CSF group (BG), bortezomib was given on day1 and G-CSF (250 μg/kg subcutaneously) was given on days 2, 3, 4, and 5. In G-CSF-bortezomib group (GB), G-CSF was given on days 2, 3, 4, and 5 and bortezomib was given on day 5. G-CSF (G) control group received G-CSF on days 2, 3, 4, and 5. In bortezomib-AMD3100 group (BA), bortezomib was given at baseline and AMD3100 (5 mg/kg subcutaneously) was given 15 h after bortezomib. In PBS-AMD3100 control group (PA), bortezomib at baseline in BA group was substituted with PBS. In cyclophosphamide-G-CSF group (CG), cyclophosphamide (200 mg/kg intraperitoneal) was given at baseline and G-CSF was given on days 2, 3, 4, and 5. In cyclophosphamide-bortezomib-G-CSF group (CBG), bortezomib at baseline was added to CG experiment.
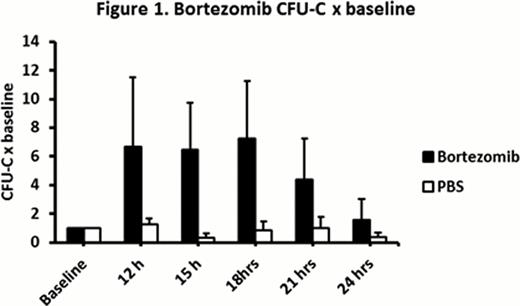
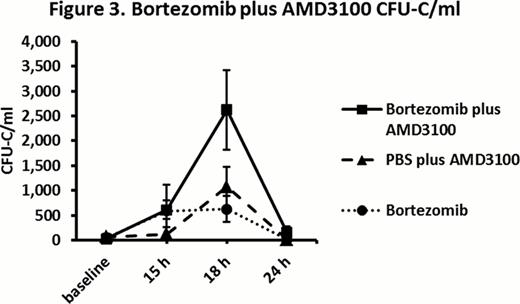
Bortezomib compared with PBS in B6 mice resulted in a significantly higher CFU-C 12 h to 18 h after baseline injections (mean peak CFU-C: 680/ml vs. 100/ml respectively, fold increase in CFU-C: 6.8 vs. 0.8 respectively, P = 0.0002) (Figure 1). In bortezomib group, CFU-C remained at peak from 12 h to 18 h and returned close to baseline 24 h after bortezomib. White blood cell (WBC) peak of 1.5 fold over baseline was observed 12 h to 15 h after bortezomib. There was no statistically significant difference in bortezomib HSPC mobilization in non-splenectomized vs. splenectomized mice (mean peak of 580/ml vs. 550/ml respectively, P = 0.89). In VLA4KO experiments, there was no statistically significant difference in peak CFU-C in bortezomib group versus PBS group (mean 730/ml vs. 590/ml respectively, P = 0.18) suggesting no HSPC mobilization effect for bortezomib in VLA4KO mice (Figure 2). In bortezomib plus G-CSF (BG) experiments, peak CFU-C on day 6 in BG group was significantly higher than G-CSF group (mean peak CFU-C: 4700/ml vs. 2400/ml respectively, P = 0.005). In G-CSF plus bortezomib (GB) experiments, peak CFU-C on day 6 in GB group was significantly higher than G-CSF group (mean peak CFU-C: 5600/ml vs. 2400/ml respectively, P = 0.001). There was no statistically significant difference in peak CFU-C in BG vs. GB groups (P = 0.28). In bortezomib plus AMD3100 (BA) vs. PBS plus AMD3100 (PA) experiments, peak CFU-C 3 h after AMD3100 in BA group was significantly higher than PA group (peak CFU-C: 2600/ml vs. 1100/ml respectively, fold increase in CFU-C: 105 vs. 12.5 respectively, P = 0.01) (Figure 3). In cyclophosphamide-G-CSF (CG) vs. cyclophosphamide-bortezomib-G-CSF (CBG) chemomobilization experiments, CBG compared to CG resulted in a trend toward higher CFU-C peak on day 6 (23500/ml vs. 21000/ml respectively, P = 0.49) and higher CFU-C on day 7 (14200/ml vs. 8700/ml respectively, P = 0.03).
Bortezomib is a potent HSPC mobilizer drug, augment AMD3100 and G-CSF mobilization in at least an additive fashion, and increase and extend chemomobization effect of cyclophosphamide. Bortezomib mobilization mechanism probably involves VLA4/VCAM-1 axis. Bone marrow rather than spleen is the source of HSPC's mobilized by bortezomib.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.