Abstract
Abstract 607
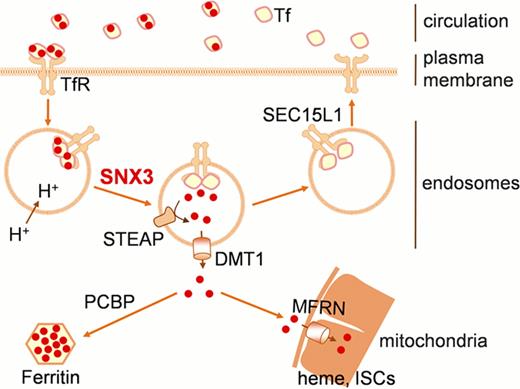
Developing erythrocytes acquire large amounts of iron through the transferrin (Tf) cycle for heme synthesis. The Tf cycle involves unidirectional transport of transferrin-transferrin receptor 1 (Tf-TfR1) complexes from the plasma membrane to the early and recycling endosomes (Figure). Besides the requirement for the basic trafficking machinery, specific sorting molecules exist to ensure the efficient re-cycling of Tf-TfR1 complexes and targeted iron delivery. The trafficking of TfR1 from recycling endosomes to the cell surface was shown to be mediated by Sec15L1, an exocyst component, as its mutation causes anemia in the hemoglobin deficit (hbd) mouse. The sorting mechanisms responsible for earlier trafficking steps in intracellular transferrin cycle, however, are poorly understood.
Here we report that sorting nexin 3 (SNX3), a cargo-specific retromer component, facilitates the endocytic recycling of TfR1, and thus, is required for the proper delivery of iron to erythroid progenitors for heme synthesis (Figure). Snx3 is highly expressed in hematopoietic tissues of zebrafish and mouse. Morpholino-mediated knockdown of snx3 in zebrafish embryos leads to a profound anemia. shRNA silencing of Snx3 in mouse primary fetal liver cells and mouse Friend erythroleukemia (MEL) cells inhibits the production of hemoglobin. We demonstrate that these defects are due to impaired transferrin-mediated iron uptake and delivery to the mitochondria. The impaired iron assimilation can be complemented with non-transferrin bound iron chelates, such as Fe-SIH (salicylaldehyde isonicotinoyl hydrazone). Furthermore, we show that SNX3 may act through direct physical interaction with TfR1 to sort Tf-TfR1 complexes to the recycling endosomes.
Our data from genetic, biochemical, and chemical biological studies collectively show that SNX3 regulates TfR1 trafficking and iron homeostasis in developing erythrocytes. The identification of SNX3 as an essential co-regulatory protein that regulates Tf-mediated iron delivery for heme synthesis provides a new genetic tool for exploring human disorders of iron metabolism, such as the hypochromic anemias, and erythropoiesis.
* Cell Metabolism (in revision).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.