Abstract
Abstract 797
Brentuximab vedotin (BV) is an anti-CD30 chimeric antibody conjugated by a protease-cleavable linker to the microtubule-disrupting agent monomethyl auristatin E. BV received accelerated FDA approval in 2011 for relapsed Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL). While CD30 expression of malignant cells in HL and ALCL is uniform, in mycosis fungoides/Sezary syndrome (MF/SS), the CD30 expression is more variable. Given the tumor selectivity and possible bystander effects, we explored the clinical activity of BV in this group of patients with variable CD30 expression and assessed for correlation of CD30 expression and response.
Patients with age 18 or older, MF/SS stages IB-IV, ECOG performance status 2 or lower, and with at least 1 prior systemic therapy were enrolled in this investigator-initiated, phase II, single-arm exploratory study. CD30 expression levels in the skin (proportion of total lymphoid infiltrate) were evaluated by routine immunohistochemistry (IHC) using the BerH2 antibody. Subjects, irrespective of pre-treatment CD30 staining, were treated with up to 8 cycles of brentuximab vedotin (1.8 mg/kg) administered every 3 weeks; optional extension of up to an additional 8 cycles was allowed in responders with ongoing clinical improvement. Global response was determined according to consensus response criteria in MF/SS. CD30 expression and clinical response data were confirmed by independent review.
19 subjects were consented and all received at least one dose of BV; median age was 59 (range 20–88) with a median of 4 prior systemic therapies (range: 1–13), with the majority having advanced disease and/or adverse prognostic risk factors (17/19 Stage IIB or greater, 13/19 large cell transformation (LCT), 8/19 folliculotropic MF (FMF), and 3/19 both LCT and FMF). Pre-treatment IHC CD30 expression (% positive of total lymphoid infiltrate) in skin biopsies was used to define three subject groups: < 10% (n=7); 10–50% (n=10); >50% (n=2).
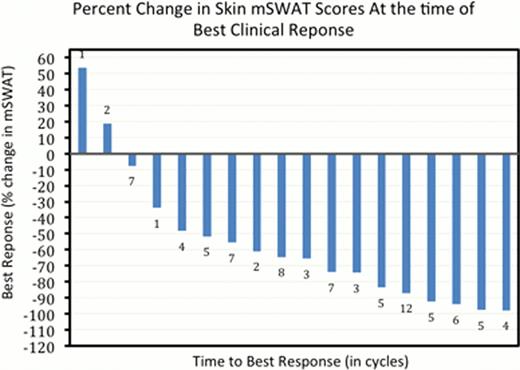
The overall response rate was 68% (13/19). Median best mSWAT reduction (response measure in the skin) was 65%. Responses were observed in all clinical stages: Stage IB (2/2 PR), Stage IIB (10/11 PR, 1/11 SD), Stage IVA/B (1/6 PR, 1/6 SD, 4/6 PD). Median time to response was 6 weeks (range 3–18). At 25 weeks, Kaplan-Meyer estimate shows 74% of responses are continuing and 73% are progression-free at time of analysis with median study follow-up time of 36 weeks (range 6–55). The median event free survival, with event defined by progressive disease, early study termination, or initiating another significant treatment was 27 weeks (range 5–47+). Related grade 1 or 2 adverse events (AEs) occurring in >20% were peripheral neuropathy (78%), fatigue (61%), decreased appetite (28%), and nausea (22%). Grade 3–4 related AEs included rash (n=3), neutropenia (n=2), and single reports of lymphocytosis, peripheral neuropathy, pruritus, pneumonia, hyperglycemia, sepsis, acute renal failure, leukopenia, and thrombocytopenia. One death was reported due to respiratory failure in the patient with pneumonia. Time to development of neuropathy was median 14 weeks (range: 6–39) and the median time to resolution or improvement in neuropathy (from onset of neuropathy) was 24 weeks (range 6–46+).
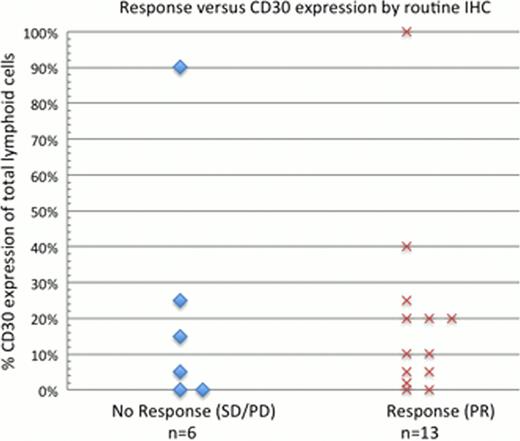
Pre-treatment CD30 quantitative image analysis (Nuance imaging system, CRi, Woburn, MA) staining data were available for 31 biopsy samples from 16 of 19 subjects. Evaluation by quantitative image analysis detected CD30 staining of lymphoid cells in all samples including 12 interpreted as negative by routine IHC, as well as 3 from tumors that developed while on BV. CD30 expression levels did not correlate with clinical response as assessed by routine IHC (p=0.17) or image analysis (p=0.74).
Our exploratory study demonstrates significant clinical activity of brentuximab vedotin in heavily pre-treated MF with mostly grade 1/2 related AEs. Clinical responses were observed in those with all levels of CD30 expression. Responses in subjects with zero to low CD30 expression by routine IHC may be in part due to low sensitivity of standard IHC detection of drug target. This study reports the successful utility of multispectral image analysis to demonstrate low levels of target expression.
Off Label Use: Brentuximab vedotin is not approved for use in mycosis fungoides / Sezary syndrome. BV is an anti-CD30 antibody conjugated to the anti-tubulin inhibitor MMAE. It is approved for use in relapsed Hodgkin Lymphoma and systemic anaplastic large cell lymphoma. Advani:Seattle Genetics: Research Funding. Nagpal:Seattle Genetics: Consultancy. Horwitz:Seattle Genetics: Consultancy, Research Funding. Kim:Seattle Genetics: Consultancy, Honoraria; Millenium Pharmaceuticals: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.