In this issue of Blood, Yang et al have demonstrated that the therapeutic activity of a targeted therapy, the tyrosine kinase inhibitor (TKI) dasatinib, unexpectedly depends on antitumor T-cell responses that are strongly potentiated by immuno-stimulation (agonist anti-OX40).1
Molecular targeted therapy inhibits tumor development by blocking oncogenic signaling molecules selectively activated in cancer cells. Targeted therapies have generated major excitement in the past decade. Mutations in c-KIT, a proto-oncogenic TK receptor, are involved in several cancers including gastrointestinal stromal tumor (GIST), melanoma, and mastocytoma.1 Although c-KIT inhibitors have improved the prognosis, most patients will still relapse due to the emergence of secondary mutations and drug-resistant tumor clones. This demonstrates the limitation of targeted therapy as single agents, and suggests the need for combination therapies.
Yang et al report that in the c-KIT mutant P815 mastocytoma the therapeutic efficacy of the TKI dasatinib substantially relies on components of the immune response. They demonstrate that the combination of dasatinib with immuno-stimulation leads to a potent therapeutic synergy resulting in complete tumor clearance in treated mice (see figure). Indeed, targeted and immune-based therapies eradicate cancer cells by different mechanisms and immunotherapy may be the only approach to elicit cancer-specific adaptive immune memory and long-term protection against relapse and metastases.
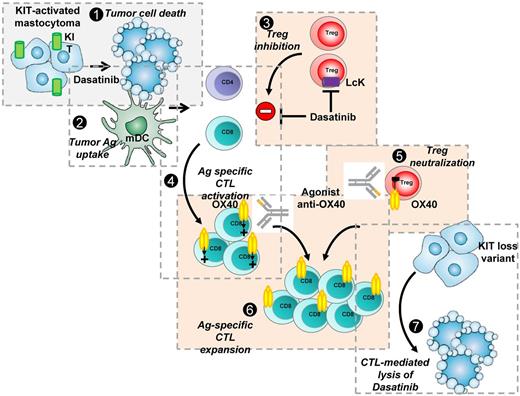
Potent synergy between KIT-targeted inhibitor dasatinib and agonist anti-OX40 mAb to eradicate P815 KIT-mutated mastocytoma tumors. (1) P815 KIT-mutated mastocytoma are sensitive to dasatinib leading to tumor cell death. (2) Cell debris are phagocytosed by DCs. (3) Dasatinib blocks Treg expansion possibly through LcK inhibition. (4) This results in CD4 T cell–dependent, tumor antigen–specific CD8/CTL activation and proliferation through antigen presentation by DCs. (5) Agonist OX40 mAb blocks Treg suppressive function. (6) Antigen-specific CTLs are OX40+ and engagement of OX40 leads to strong amplification of the activated antigen-specific CD8 pool and long-term maintenance of CD8 memory. (7) Tumor antigen–specific CD8 memory T cells detect and eradicate remaining tumor cell clones, even KIT loss variant emerging during targeted therapy, resulting in complete tumor eradication.
Potent synergy between KIT-targeted inhibitor dasatinib and agonist anti-OX40 mAb to eradicate P815 KIT-mutated mastocytoma tumors. (1) P815 KIT-mutated mastocytoma are sensitive to dasatinib leading to tumor cell death. (2) Cell debris are phagocytosed by DCs. (3) Dasatinib blocks Treg expansion possibly through LcK inhibition. (4) This results in CD4 T cell–dependent, tumor antigen–specific CD8/CTL activation and proliferation through antigen presentation by DCs. (5) Agonist OX40 mAb blocks Treg suppressive function. (6) Antigen-specific CTLs are OX40+ and engagement of OX40 leads to strong amplification of the activated antigen-specific CD8 pool and long-term maintenance of CD8 memory. (7) Tumor antigen–specific CD8 memory T cells detect and eradicate remaining tumor cell clones, even KIT loss variant emerging during targeted therapy, resulting in complete tumor eradication.
The US Food and Drug Administration's approval of anti–CTLA-4 monoclonal antibody (mAb) in the United States for the treatment of melanoma and the recent successes obtained in clinical trials using anti-PD1 and PD-L1 mAbs2 demonstrate the potency of immunotherapy strategies. The clinical development of these immunomodulatory antibodies represents a paradigm shift in cancer therapy: for the first time the immune system is targeted rather than the tumor itself.
Although the immune system has been demonstrated to contribute to the activity of certain chemotherapies,3 most are also affecting immune cells activated during the tumor cell destruction. Thus, the combination of tumor-targeted therapy with immuno-stimulation is an attractive approach as demonstrated by Yang and colleagues.1 However, it is important to stress that targeted therapies may have previously undescribed effects that might alter immune cells.
Indeed, the immune effect of dasatinib reported here might operate via several mechanisms (see figure). First, by inducing targeted tumor cell death, dasatinib might lead to immunogenic tumor debris as described for other chemotherapies.3 Second, the authors report here that dasatinib in vivo treatment results in selective decrease of regulatory T cells (Tregs), suggesting dasatinib is acting in part by reversing immuno-suppression. Indeed, in a recent study imatinib was also shown to act on another immuno-suppressive pathway, the IDO enzyme activity.4 Because TKIs target multiple kinases, action on immune-related kinases might contribute to the observed immuno-modulatory effect of dasatinib. It has been reported that dasatinib inhibits T-cell expansion through blocking Lck,5 a critical kinase in TCR signaling. The authors suggest that Tregs are more sensitive to dasatinib than effector T cells, suggesting a higher Lck dependence of Tregs. Importantly, different doses and schedules of drug administration might have opposite consequences on immunity, with strong CTL response with short-term dasatinib treatment, but immunosuppression with long-term treatment.1 This supports the use of intermittent high-dose pulse dasatinib treatment.
Other immune-mediated activity of TKIs has been previously reported; in particular, imatinib has been reported to increase NK function likely through modulating NK/DC cross-talk.4 In addition, dasatinib has been reported to increase NK reactivity in chronic myeloid leukemia patients6 and in vitro NK cell expansion.
These observations argue for the need for comprehensive understanding of activities of targeted therapies on the immune system for optimal use in combination with immuno-stimulation.
Yang et al used agonistic anti-OX40 mAb to increase antitumor immunity, resulting in potent therapeutic synergy with dasatinib (see figure).1 Recently, the combination of imatinib and anti–CTLA-4 was reported to induce tumor clearance in a spontaneous model of GIST.4 Agonist anti-OX40 has been reported to increase immunity through both neutralization of Treg function and increased resistance of T effectors to suppression7 and is currently under clinical development.8 Although OX40 engagement leads to T-cell expansion, Treg numbers also increased in the present study. Thus, it will be critical to analyze the outcome of the antitumor immune response during in vivo decay of agonist anti-OX40 levels: would the neutralized Tregs take control again?
There is no doubt that the combination of targeted therapy and immunotherapy will be studied in clinical trials and will change clinical management. There is already an ongoing clinical trial in melanoma evaluating the combination vemurafenib (Braf inhibitor) plus anti–CTLA-4. Other immune strategies currently being studied include mAbs interfering with immune checkpoints (OX40, CD137, GITR) or endowed with Ab-dependent cell cytotoxicity capacities, vaccines (long peptides, poxviruses, synthetic mRNA), cytokines (IL-7), and vaccine adjuvants (TLR agonists).9 Among the immune checkpoints, strategies directly targeting Tregs are also under development.10 OX40 is one such target that neutralizes Treg function but not expansion, but will require careful monitoring during development.
In conclusion, the recent positive results of clinical trials with novel immuno-active drugs as well as the unexpected finding of a positive interaction between immunotherapy and targeted therapy should open a new era for the personalized immunotherapy of cancer.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■