In this issue of Blood, Christoffersson et al provide data indicating the existence of a new neutrophil subset with angiogenic characteristics.1
Until recently, neutrophils were thought to be a homogeneous cell type that rapidly arrive at sites of infection or injury, eradicate infiltrating microbes, die, and are taken up by macrophages. However, recent reports indicate that there might be multiple neutrophil subpopulations, similar to monocytes, with not only pro-inflammatory but also anti-inflammatory properties.2-4 However, whether this is the same neutrophil at different stages of its life cycle, whether this is the same neutrophil just stimulated differently, or whether these are truly bona fide neutrophil subtypes remains debatable. If correct, however, multiple neutrophil subtypes could have significant implications for conditions ranging from chronic inflammation and cancer to organ transplant survival.
In the current study, Christoffersson and coworkers add additional evidence that multiple neutrophil subtypes exist, and propose a subset of neutrophils with pro-angiogenic properties, which are high in CXCR4, recruited to newly grafted tissues by VEGF-A, and release MMP-9 (matrix metalloproteinase 9) allowing for graft revascularization (MIP-2–recruited neutrophils had low levels of MMP-9 and CXCR4; see figure).1 Although neutrophil subsets have been postulated to exist,2-4 the prevailing criticism was that the neutrophil may be plastic, and as such could adapt to its environment. In vitro experiments suggested that neither VEGF-A nor MIP-2 could induce these phenotypic changes, suggesting 2 distinct populations before entry into tissues.1 In addition to identifying pro-angiogenic neutrophils, this study also suggests new functions for VEGF-A (in neutrophil recruitment) and MMP-9 (in revascularization).
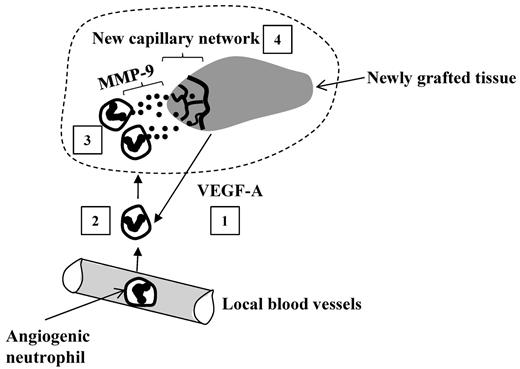
Functioning of angiogenic neutrophils within the graft. The grafted tissues release VEGF-A that recruits a specific subset of angiogenic neutrophils from blood into the graft (1). The extravasated neutrophils (2) infiltrate the graft where they release MMP-9 (matrix metalloproteinase 9; (3) facilitating angiogenesis: development of a new capillary network (4) rescuing the graft from hypoxia.
Functioning of angiogenic neutrophils within the graft. The grafted tissues release VEGF-A that recruits a specific subset of angiogenic neutrophils from blood into the graft (1). The extravasated neutrophils (2) infiltrate the graft where they release MMP-9 (matrix metalloproteinase 9; (3) facilitating angiogenesis: development of a new capillary network (4) rescuing the graft from hypoxia.
Neutrophils are the first immune cells to arrive at the inflammatory site. They leave blood vessels, passing barriers of endothelial cells, pericytes, and basement membrane, a complex and continuous structure composed of extracellular matrix (ECM) proteins. Digestion of ECM can be achieved by neutrophils through the release of proteases, including MMP-9. However, reports on the involvement of MMP-9 in extravasation are ambiguous. Some of the confusion may result from overlapping MMP substrate specificities and thus compensation. Alternatively, MMP-9 is dispensable for neutrophil extravasation in vivo, given that the cells actively choose ECM-poor regions of basement membrane for migration.5 In this study, despite a 10-fold increase in MMP-9 content in angiogenic versus inflammatory neutrophils, both subtypes infiltrated tissue equally well, further supporting the view that MMP-9 is dispensable for extravasation. During infectious inflammation, neutrophils are the big kids at the playground, responsible for elimination of invading pathogens and debris clearance. During sterile inflammation (eg, caused by hypoxia), they infiltrate injured tissue and contribute to wound debris removal.6 This latter function may be achieved by MMP-9 degrading intracellular matrix (ICM) proteins released from dead/damaged cells.7 Finally, a cell type must then come in and help with revascularization and healing. Whether only inflammatory neutrophils enter infectious sites while both inflammatory and angiogenic neutrophils enter sterile injury to help with progression of inflammation and restitution remains unclear, but the current study may support such a scenario.
Christoffersson and colleagues report that neutrophil MMP-9 aids in the development of new vasculature in transplanted pancreatic islets. Mice genetically deprived of this enzyme have diminished revascularization during the first days and, although revascularization eventually occurs, it does so in a longer time frame and the vascular architecture of the new capillaries is significantly altered.1 That revascularization occurs at all suggests potential compensatory action of other proteases, but it is clear that MMP-9 is responsible for proper angiogenesis.
The involvement of neutrophils in angiogenesis was reported previously in a corneal injury model.8 This process also involved VEGF, but neutrophils engaged in this process were not phenotyped. In the current study Christoffersson and coworkers demonstrate that angiogenic neutrophils have pre-existing features: high expression of CXCR4 and MMP-9.1 While the latter is not surprising in light of MMP-9 involvement in vascularization, the importance of the chemokine receptor CXCR4 is interesting as its main ligand CXCL12 (SDF-1) was shown not to be involved in the recruitment of angiogenic neutrophils. In contrast, neutrophils were observed to infiltrate tissues after application of VEGF-A, and pancreatic islets from VEGF-deficient mice were unable to efficiently recruit neutrophils.1 It is not known whether VEGF-A is able to directly recruit neutrophils.
It remains unexplained what the role of CXCR4 would be if not for migration. Previous studies have shown that CXCR4 is required for neutrophil release from the bone marrow.9 Although this receptor is necessary for maximal neutrophil mobilization into blood, it is not essential for further neutrophil emigration to sites of inflammation. This might explain why, in the current model, CXCL12 was not necessary for neutrophil migration to the grafted tissue. On the other hand, the receptor is also important, although dispensable, for homing of aged, senescent neutrophils to the bone marrow and other organs.9 If so, the expression of CXCR4 on the angiogenic neutrophils might facilitate their clearance once they fulfill their function. This would require that the neutrophils leave the sterile injury, perhaps re-entering the vasculature, something proposed in zebra fish embryos.10
The current study by Christoffersson et al characterizes a new population of angiogenic neutrophils. Clinically, β cell replacement of the pancreatic islets is a promising therapeutic approach for a cure of type 1 diabetes, and from this point of view the role of neutrophils presented in this work is favorable; however, these same angiogenic neutrophils might also facilitate vascularization of tumors leading to the outgrowth of cancer tissues. It is this dual role of angiogenic neutrophils that opens exciting therapeutic possibilities. By regulating their recruitment to specific sites, it may be possible to either help support the revascularization of transplanted tissues, helping to ensure their survival, or to starve tumors of their blood supply, leading to hypoxia and tumor cell death.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■