Abstract
Risk factors for deep-vein thrombosis have been shown not to be always the same as for pulmonary embolism. A well-known example is the factor V Leiden (FVL) paradox: the FVL mutation poses a clearly higher risk for deep-vein thrombosis (DVT) than for pulmonary embolism. We aimed to expand this paradox and therefore present risk estimates for several established risk factors for DVT and pulmonary embolism separately. When such separate risk estimates could not be retrieved from the literature, we calculated these risks in our own data, a large population-based case-control study on venous thrombosis (the MEGA study). Our results showed that the FVL paradox can be broadened (ie, the risk factors oral contraceptive use, pregnancy, puerperium, minor leg injuries, and obesity have an effect comparable with FVL). Furthermore, we found that pulmonary conditions, such as chronic obstructive pulmonary disease, pneumonia, and sickle cell disease, were risk factors with an opposite effect: a higher risk of pulmonary embolism, but little or no effect on DVT. These findings suggest that pulmonary embolism and DVT may not always have the same etiology, and encourage unraveling this phenomenon in further studies.
Introduction
Venous thrombosis (VT) is a disease with 2 main manifestations, pulmonary embolism (PE) and deep-vein thrombosis (DVT). With an incidence of 1 to 3 per 1000 per year,1 VT causes significant morbidity and mortality. Risk factors for VT have been thoroughly studied and can be divided into genetic and acquired risk factors. Surprisingly, considering that PE and DVT are regarded as the same disease, it appears from several studies that risk factors for PE are different from those for DVT. The most prominent difference has been found for factor V Leiden (FVL). DVT patients are more likely to carry the FVL mutation than PE patients.2 Compared with control subjects, the relative risks of DVT and PE, expressed as odds ratios, were 4.5 (95% confidence interval [CI], 3.8-5.3) and 1.7 (95% CI, 1.3-2.2), respectively.3 This differential effect of FVL has become known as the FVL paradox.2,4 Many studies from different countries have confirmed the paradox since the concept was put forward in 1996.3,5-12
When we consider other risk factors, some also appear to have differential effects on the risk of PE and DVT (eg, sickle cell disease, which was found to be associated with a higher risk of PE than DVT).13 With this in mind, the question arises whether thinking of venous thromboembolic disease as one entity is always justified.
We aimed to give an overview of currently known risk factors for VT and to assess risk estimates for PE and DVT separately. When the literature search did not yield risk estimates separately for DVT and PE, we calculated these odds ratios in our own data (ie, a large population-based case-control study on causes of VT, the MEGA study).
Methods
For this overview, 2 data sources have been used. First, we performed a systematic review, including studies obtained from an extensive PubMed search on all common risk factors for PE and DVT, which described separate risk estimates for both disease entities. Second, when we could find in the literature no or only one report for a specific risk factor providing separate effects, we calculated these risk estimates using data from a large population-based case-control study (MEGA).
Data selection for the systematic review
A selection was made from recent literature to list common genetic and acquired risk factors for VT.14 Coagulation factors were not included in this overview, as they were considered not to be primary risk factors but rather mediators of disease. For these factors, a PubMed search was performed for articles using the following criteria: papers should present risk estimates such as relative risk (RR), odds ratio (OR), or incidence rate ratios (IRR) with 95% CIs. Estimates should be presented for DVT and for PE separately, and the same reference group had to have been used to allow comparison of relative risks. When articles presented crude data with the possibility to calculate the afore-described effect measures, they were also included in this overview. (Re)calculated data were marked in the tables. We included data from population-based studies and excluded family studies. A detailed description of our search strategy can be found in the Appendix. In addition to the articles that were found by the PubMed literature search, we used cross-references to extend the search.
VT events had to have been either confirmed by an imaging modality, such as compression ultrasonography, CT pulmonary angiography, or ventilation-perfusion scanning; or diagnosed as International Statistical Classification of Diseases codes.
All included articles had to describe whether they studied PE alone or PE with or without concomitant DVT. Meta-analyses presenting pooled effect measures from different populations were not included.
Defining criteria for differences between risk factors
When we interpreted the effect of a risk factor for PE and DVT, we compared relative risks. However, for a fair comparison, the absolute risk of PE and DVT has to be taken into account because their baseline incidences are different. The incidence of DVT in the population is generally twice as high as the incidence of PE.1 Therefore, when a risk factor adds a similar absolute risk to the baseline risk of PE and DVT, we expect that the RR (DVT) is smaller than the RR (PE). For example, when the baseline risk for DVT is 2/1000 per year and for PE 1/1000 per year, and a risk factor adds 2/1000 cases per year, the relative risk for DVT will be 2 (4/2) and that for PE will be 3 (3/1). The RR(PE)/RR(DVT) is 1.5 in this case.
So, for risk factors with a RR more than 1, the following conditions hold true (Appendix 1): If 1 ≤ RR(PE)/RR(DVT) < incidence (DVT)/incidence (PE), the risk factor is considered to have a similar effect for DVT as for PE. This can be rewritten as 1 ≤ RR(PE)/RR(DVT) < 2. If RR(PE)/RR(DVT) < 1 or RR(PE)/RR(DVT) ≥ 2, the effect of the risk factor on the incidences of DVT and PE is considered not to be similar.
To illustrate the applicability of this criterion, we tested it on all 10 studies into the separate effect of FVL where it was met 8 times. In case a study presented inverse RRs (ie, the protective effect of a risk factor was given), we first converted these RRs to their inverse to be able to use our criterion.
When 2 or more studies were available on a certain risk factor, we considered pooling the reported risk estimates for DVT and for PE. However, after studying the data in more detail, we decided that pooling was undesirable because of heterogeneity of the study designs (case-control vs cohort studies), heterogeneity in age distribution of the study populations, and the occurrence of reports of both protective effects and harmful effects for one risk factor. When 2 or more studies were available, we considered a risk factor to have a different effect on DVT and PE whether the risk of DVT was consistently higher than that of PE, or vice versa, according to the previously defined criterion.
Design of the MEGA case-control study
The MEGA study is a population-based case-control study that included consecutive patients with a first event of DVT or PE.3 Patients 18 to 70 years of age provided a questionnaire and DNA or plasma. Inclusion of patients took place between 1999 and 2004 from 6 anticoagulation clinics in The Netherlands. Controls were either partners of the patients, or random digit dialing controls. A total of 5183 cases and 6297 controls were enrolled in the MEGA study. A total of 4751 cases and 5916 controls completed the questionnaire. In 4483 cases and 4880 controls, DNA was available (from either blood or buccal swabs). For 2469 cases and 2940 controls, blood samples were available (number limited for logistic reasons).
A logistic regression model was applied to calculate odds ratios for DVT patients versus controls and PE patients versus controls. In addition, patients with both PE and concomitant DVT were analyzed as a third group compared with controls. For each different risk factor, prespecified covariables were added to the regression model, thereby adjusting for confounding.
Results
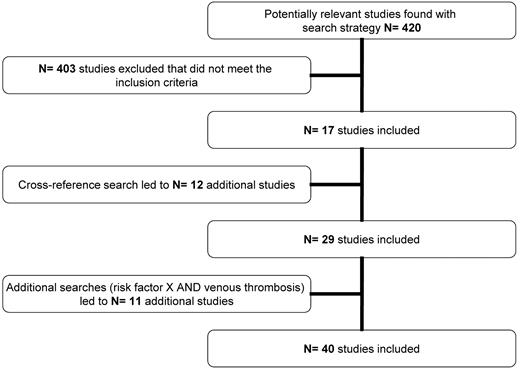
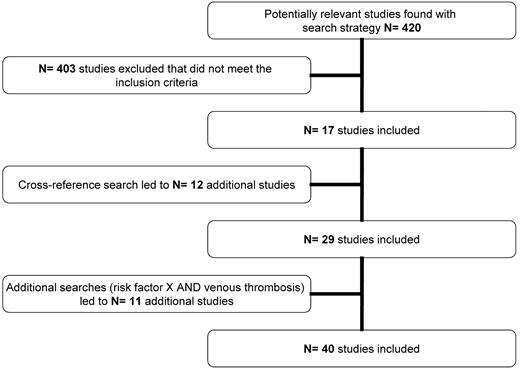
We initially found 420 articles of which, after applying our inclusion criteria and cross-referencing, 40 articles remained to be included (Figure 1; supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For several risk factors, however, data from the literature search were lacking. The MEGA study did not contain data for all risk factors either. Table 1 shows the risk factors that were included in our search and indicates which could be used for this review.
General and morphometric risk factors
General and morphometric risk factors (Table 2) are as follows:
Age.
Age is one of the strongest risk factors for VT and has been described as such in several population-based cohort studies.1,15-18 Table 2 shows 4 cohort studies that presented incidence rates for DVT and PE separately. The increase with age holds true for both PE and DVT patients in these studies. Overall, these 4 studies consistently show that age is associated with a higher risk estimate for PE than for DVT. However, the difference between PE and DVT is such that RR(PE)/RR(DVT) is not more than or equal to 2 (ie, the criterion for a definite difference between the 2 risk estimates is not met).
Sex.
In some studies, male sex has been described as a risk factor for VT,16,18-20 whereas other studies found VT risk to be slightly higher in women.1,17 Table 2 shows 5 studies that presented data for DVT and PE separately. Because of the heterogeneity of these findings, we were not able to conclude that sex has a different effect on DVT than on PE.
Ethnicity.
Studies presenting differences in VT incidence by ethnicity have been mainly performed in the United States.21-23 For Asians and Hispanics, a lower incidence was found than for whites, whereas blacks seem to be at higher risk than whites. Table 2 presents RRs for blacks, Asians, and Hispanics with whites as a reference group. From these data it appears that blacks tend to develop PE more often than DVT, compared with whites.
Height.
Increasing body height has been shown to increase the risk of VT.24,25 We found no studies that assessed body height as a risk factor for PE and DVT separately.
Results from the MEGA case-control study on the effect of body height showed a subtle increase in risk with increasing body height for DVT and PE to the same extent (L.E.F., A.v.H.V., F.R.R., and S.C.C., Body height, mobility, and risk of first and recurrent venous thrombosis, manuscript in preparation; Table 2).
BMI.
Overweight, defined as a BMI more than or equal to 25 kg/m2, and obesity, defined as a BMI more than or equal to 30 kg/m2, have been documented as risk factors for VT in several studies. Two studies presented data for PE and DVT separately.26,27 The first study used hospital discharge data from the National Hospital Discharge Survey to assess the risk of obesity on VT.26 The RR of DVT was 2.50 (95% CI, 2.49-2.51), whereas the RR of PE was 2.21 (95% CI, 2.20-2.23). Data from the second study (MEGA) showed a clear dose-response effect for increasing BMI and VT risk.27 In summary, both studies consistently showed that BMI was a stronger risk factor for DVT than for PE, and the criterion was met (RR(PE)/RR(DVT) < 1).
Transient acquired risk factors (Table 3) are as follows:
Immobilization/stasis.
Stasis of venous blood is one of the 3 components of Virchows triad and is as such a traditional risk factor for VT. We studied travel and paralysis as causes of immobilization and stasis, and their association with risk of DVT and PE.
In a multicenter cohort study performed at emergency departments in the United States, immobilization resulting from neurologic causes was associated with a 10-fold increased risk of PE and an 8-fold increased risk of DVT. Travel did not seem to contribute to VT risk in this cohort.28
In the MEGA study, the effect of various modes of travel was studied.29 A flight of more than 4 hours gave a 3-fold increased risk of DVT (95% CI, 1.3-7.1). Travel by car, bus, or train gave an OR of 1.9 (95% CI, 1.1-3.2) for DVT. For PE patients the OR for air travel was 0.64 (95% CI, 0.25-1.6) and for other modes of travel the OR was 4.0 (95% CI, 1.5-10.7). In summary, no consistent differences in risk of DVT or PE were seen regarding immobilization and stasis.
Surgery.
In the Million Women Study, different types of surgery and thrombotic outcomes were studied.30 Risks were similar for PE and DVT, although short-term risk for PE seemed to be higher than for DVT. We calculated DVT- and PE-specific RRs using person-years from Table 2 from the original article. For the first 6 weeks postoperatively, the RR for DVT was 31.7 (95% CI, 28.4-35.3), whereas for PE the RR was 41.5 (95% CI, 36.9-46.7).
In the MEGA study, surgery within one year increased the risk of isolated DVT 4-fold (95% CI, 3.5-4.6), with a similar 4.3-fold increased risk of isolated PE (95% CI, 3.7-5.1; unpublished data). Based on these 2 studies, we conclude that there is no difference in postoperative risk between DVT and PE.
Minor leg injury.
In the MEGA study, there was a stronger association with minor injuries in the leg (OR adjusted for age and sex, 5.1; 95% CI, 3.9-6.7) than with injuries in other parts of the body. The OR for PE was 2.4 (95% CI, 1.6-3.7), whereas for DVT alone the OR was 6.3 (95% CI, 4.7-8.5; Table 3).31 We conclude that minor leg injury is a risk factor that has a stronger effect on DVT than on PE as the criterion RR(PE)/RR(DVT) < 1 was met.
Oral contraceptives.
Oral contraceptive use has been identified as an important risk factor for DVT and PE since the 1960s. In the MEGA study, a different effect of oral contraceptives was found for PE (OR 3.9; 95% CI, 3.2-4.8) than for DVT (OR 6.6; 95% CI, 5.4-8.0).32
A WHO multicenter case-control study, including women from different continents, also presented point estimates for PE and DVT separately.33 For the European cases classified as “definitive” according to prespecified diagnostic standards, the OR for PE was 2.5 (95% CI, 0.95-6.8), whereas for DVT the OR was 4.1 (95% CI, 2.8-6.1). So, for European women, oral contraceptive use has a stronger effect on DVT than on PE and the criterion was met.
Pregnancy/puerperium.
Pregnancy and puerperium are the strongest risk factors for VT among young women. Three studies described the risks during and after pregnancy for DVT and PE separately.34-36
The first study, a cohort from the United States, showed an IRR of 0.23 (95% CI, 0.07-0.71) for PE and an IRR of 1.9 (95% CI, 1.3-2.7) for DVT during pregnancy.36 For puerperium an IRR of 3.5 (95% CI, 2.2-5.6) for PE and an IRR of 7.6 (95% CI, 5.5-10.6) for DVT was found.
Another cohort study, from Glasgow, assessed the incidence of VT among 72 201 deliveries.34 They found an incidence of 0.50 DVTs per 1000 deliveries during pregnancy and 0.21 DVTs per 1000 deliveries puerperal. For PE, an incidence of 0.07 per 1000 deliveries was found antenatal and an incidence of 0.08 per 1000 deliveries puerperal.
The MEGA study found an OR of 2.3 (95% CI, 1.0-5.2) for PE and an OR of 7.8 (95% CI, 4.1-15.0) for DVT during pregnancy. Postpartum an OR of 34.4 (95% CI, 13.3-88.5) for PE and an OR of 72.6 (95% CI, 30.1-175.4) for DVT was found.35 All 3 studies consistently showed a higher risk of DVT than of PE for women during pregnancy and postpartum period, and the criterion was met.
Exercise.
The few studies about VT and sports or physical activity show conflicting results; some show a slightly increased risk and some a slightly decreased risk.37-40 One study separated the risk for DVT and PE.40 For PE an OR of 0.54 (95% CI, 0.46-0.64) was found, and for DVT an OR of 0.76 (95% CI, 0.67-0.86) for those who exercise at least once a week. From these findings, we conclude that there is no difference in the protective effect of exercise against PE and DVT.
Smoking.
Smoking is one of the major risk factors for arterial thrombosis. However, the association with risk of VT is less pronounced.41 Previously published data from the MEGA study reported the risk of DVT and PE separately for smokers.42 For current smokers an OR of 1.5 (95% CI, 1.3-1.7) was found for patients with DVT and a similar OR of 1.5 (95% CI, 1.3-1.8) was found for PE. For former smokers, an OR of 1.2 (95% CI, 1.0-1.4) was found for DVT and an OR of 1.4 (95% CI, 1.2-1.6) for patients with PE. Overall, no differences between risk of DVT and PE were seen.
Alcohol.
Results from the MEGA study showed decreased risks in case of moderate alcohol use for PE and DVT separately.43 Drinking 2 to 4 glasses a day decreased the risk of PE 0.56-fold (95% CI, 0.46-0.70) and of DVT 0.74-fold (95% CI, 0.63-0.88). Therefore, we conclude that moderate alcohol has similar effects on DVT and PE.
Chronic acquired risk factors
Chronic acquired risk factors (Table 3) are as follows:
Cancer.
Cancer is one of the systemic diseases that lead to a highly increased risk of a venous thrombotic event. Of many studies published on this topic, only the MEGA study presented results separately for PE and DVT patients.44 In this study, the risk of PE in patients with cancer compared with controls (OR = 4.6; 95% CI, 3.6-6.4) was similar to the risk of DVT (OR = 4.0; 95% CI, 3.0-5.3).
COPD.
PE often occurs among patients with chronic obstructive pulmonary disease (COPD).45-47 However, the risk of DVT among these patients was not known until recently. A nested case-control study showed a 3.6-fold (95% CI, 1.3-9.7) increased risk of PE for patients with mild COPD and a 7.5-fold (95% CI, 2.4-23.7) increased risk of PE for patients with severe COPD. No increased risk of DVT was found.48 In the MEGA study, we found a 3.2-fold increased risk of PE (95% CI, 2.4-4.2) for patients with COPD and a 1.6-fold (95% CI, 1.2-2.1) increased risk of DVT for patients with COPD (L.E.F., A.v.H.V., K. W. van Kralingen, S.C.C., and F.R.R., Chronic obstructive pulmonary disease and pulmonary embolism, manuscript in preparation). In summary, according to 2 studies, COPD seems to be a risk factor for PE but hardly so for DVT. Our criterion was met in both studies.
Sickle cell trait.
Two studies assessed the risk of DVT and PE separately in patients with sickle cell disease.13,49 The first study was a cross-sectional study performed in the National Hospital Discharge Survey database, which contains hospital data from the United States.49 For PE an RR of 1.5 (95% CI, 1.4-1.6) was found and for DVT a RR of 0.75 (95% CI, 0.74-0.76) was found.
The second study was a case-control study (the GATE study).13 These investigators found a 3.9-fold (95% CI, 2.2-6.9) increased risk of PE for sickle cell trait patients and a 1.1-fold (95% CI, 0.65-1.9) increased risk of DVT. Both studies showed an increased risk of PE and no increase in risk of DVT and fulfilled the criterion.
Inflammatory bowel disease (Crohn and ulcerative colitis).
Inflammatory bowel disease has been shown to be associated with a 1.5- to 3.6-fold increased risk for VT.50-52 In one cohort study from Canada, risks for DVT and PE were presented separately. The IRR was 4.7 (95% CI, 3.5-6.3) for DVT and 2.9 (95% CI, 1.8-4.7) for PE in Crohn disease and 2.8 (95% CI, 2.1-3.7) for DVT and 3.6 (95% CI, 2.5-5.2) for PE, in ulcerative colitis.53 Therefore, no conclusions can be drawn as to a difference in risk for PE and DVT.
Kidney disease.
Kidney disease is a broad definition, but recent studies have found an increased risk of VT for most underlying causes. Again, only the MEGA study had data separately for PE and DVT. For patients with chronic kidney disease, an OR of 4.2 (95% CI, 2.4-7.2) was found for PE and of 3.3 (95% CI, 1.9-5.6) for DVT (G. Ocak, C. Y. Vossen, M. Verduijn, F. W. Dekker, F.R.R., S.C.C, and W. M. Lijfering, Risk of venous thrombosis in persons with major illnesses: results from the MEGA study, manuscript under review). According to our criteria, we found no difference in kidney disease risk estimates for DVT or PE.
Hyperthyroidism.
Thyroid dysfunction, particularly free thyroxine levels, have only recently been shown to be a risk factor for VT.54 However, only patients with a DVT of the leg were enrolled in this study and no PE patients.
Data from another case-control study (TROL study, Norway) showed a 2-fold increased risk of DVT (95% CI, 1.2-3.5) for persons with FT4 levels above the 95th percentile compared with the reference category. The risk of PE was 1.2-fold increased (95% CI, 0.5-2.7; J. Debeij, O. M. Dekkers, B. O. Asvold, S. C. Christiansen, I. A. Naess, J. Hammerstrom, F.R.R., and S.C.C., Increased levels of free thyroxine and risk of venous thrombosis in a large population-based prospective study, manuscript under review).
In the MEGA study, persons with FT4 levels more than 24pM had a 2.2-fold increased risk of PE (95% CI, 0.6-8.3), compared with the reference group (FT4 < 24pM). The risk of DVT was similar, with an OR of 2.7 (95% CI, 1.2-6.2; J. Debeij O. M. Dekkers, B. O. Asvold, S. C. Christiansen, I. A. Naess, J. Hammerstrom, F.R.R., and S.C.C., Increased levels of free thyroxine and risk of venous thrombosis in large population-based prospective, manuscript in preparation). In conclusion, no consistent differences were found in the risk of hyperthyroidism for PE and DVT.
Genetic risk factors
Genetic risk factors (Table 4) are as follows:
Blood group (non-O vs O).
In 1969, Jick described a 1.6-fold increased risk of VT (95% CI, 1.0-2.7) for a group of non-O carriers compared with blood group O carriers.55 Later studies included only DVT patients56-58 or DVT and PE patients but without outcome measures for each group separately.55,59-64 So, we could use data from only 3 studies.
The LITE study showed estimates for non-O versus O blood group carriers of 1.4 (95% CI, 1.1-1.8) for DVT as opposed to an OR of 2.0 (95% CI, 1.4-2.9) for PE, after age adjustment.65
Data from the MEGA study showed an OR of 2.3 (95% CI, 2.0-2.6) for DVT of blood group non-O versus O, whereas the risk of PE was 1.7-fold increased (OR 1.7; 95% CI, 1.4-1.9; J. Debeij, B. van Zaane, O. M. Dekkers, C. J. M. Doggen, J. W. A., Smit, A. P. van Zanten, D. P. M. Brandjes, H. R. Büller, V. E. A. Gerdes, F.R.R. S.C.C., High levels of free thyroxine increased levels of coagulation factors and the risk of venous thrombosis: results of a large population-based case-control study (MEGA-study), manuscript in preparation).
Another study that presented data for DVT and PE patients separately was performed in women registered with a VT discharge diagnosis during pregnancy or puerperium.66 Here, blood groups A and AB were associated with a similarly increased risk for DVT and PE. Because of the high baseline risk in this study (all women were either pregnant or postpartum), we did not include these results in Table 4. To conclude, we found no difference in effect estimates for PE compared with DVT.
FVL.
We have described the FVL paradox in the Introduction. Table 4 shows the studies that presented data for PE and DVT separately. Although the ORs varied between studies, the direction of the effect was consistently toward a higher risk for DVT than for PE. Of note, the risk estimate for patients with PE and concomitant DVT is generally closer to the OR for DVT than to the OR for PE.
Prothrombin G20210A.
This mutation was described in 199667 and was shown to increase the risk of thrombosis 3-fold. Its prevalence has been shown to differ between PE and DVT patients, but no clear paradox as for FVL has been described.
Results from the MEGA study showed an OR of 3.2 (95% CI, 2.4-4.2) for DVT, and an OR of 2.3 (95% CI, 1.5-3.3) for PE.3 Margaglione also found a higher risk for DVT than for PE, with an OR of 3.6 (95% CI, 2.4-5.4) and 2.1 (95% CI, 1.1-4.1), respectively.10 Another case-control study, from Bulgaria, found similar results: the prothrombin mutation was associated with an almost 3-fold increased risk of DVT, as opposed to a 1.4-fold increased risk of PE.9 One study from Spain found an increased risk for thrombotic events but no differences for DVT and PE.8 Three studies in Table 4 show a tendency for a lower relative risk in the DVT group than in the PE group.11,68,69 Of note, a case-control study conducted in China, including 369 patients, found a zero prevalence of prothrombin G20210A and FVL in their case and control group. The authors conclude that both mutations do not seem to affect Asian PE and DVT patients. We did not include this study in our table.70
In summary, the presented data do not point in one direction for the prothrombin mutation, and we conclude that there is no difference in risk of DVT and PE.
Discussion
The differential effect of the FVL mutation on PE and DVT has become known as the FVL paradox. This paradox formed the initial inspiration for this overview, in which we assessed whether differential effect sizes for DVT and PE could be confirmed for other common VT risk factors. We hypothesized that the paradox could be broadened. In the following sections, we discuss risk factors divided into 2 categories: those that we found to give a higher relative risk of DVT than PE (ie, in line with the FVL paradox) and those that had a higher risk of PE than DVT. Furthermore, we propose mechanisms for the differences we found. Finally, misclassification of DVT and PE and other limitations are addressed.
A higher risk for DVT than PE was defined as RR(PE)/RR(DVT) < 1. In addition to FVL, this held true for the reproduction-related risk factors (ie, pregnancy, puerperium, and use of oral contraceptives), as well as for obesity and minor leg injuries. The original factor for which the “paradox” is named, factor V Leiden, leads to increased activated protein C (APC) resistance. It is notable that several of the factors that also showed a higher risk for DVT than for PE also lead to increased APC resistance (ie, oral contraceptives, pregnancy, and puerperium). In an endogenous thrombin potential (ETP)–based assay, the vast majority of FVL-independent APC resistance was attributable to oral contraceptive use or pregnancy.71
A similar mechanism is likely for obesity, which also posed a higher risk for DVT than PE. In the MEGA study, APC resistance increased linearly with increasing BMI in subjects without FVL.72 (S. C. Christiansen, W. M. Lijfering, I. A. Næss, J. Hammerstrøm, A.v.H.V., F.R.R., and S.C.C., The relation between body mass index, APC-resistance and risk of venous thrombosis, manuscript under review). In addition, obesity displayed synergistic effects with other causes of APC resistance, leading to highly elevated incidences of thrombosis (ie, when jointly present with oral contraceptive use and the presence of the FVL mutation).27
So, these data suggest that APC resistance preferentially affects the risk of DVT and not of PE, and that this holds true for all upstream thrombotic causes acting via APC resistance, as FVL, obesity, pregnancy, puerperium, and oral contraceptive use. This preferential effect of APC resistance on DVT has been explained for FVL through an impaired thrombin-activable fibrinolysis inhibitor-dependent profibrinolytic response to APC, hence decreased fibrinolysis and thereby decreased embolization.73,74 Possibly this also applies to persons who are APC-resistant for other reasons.
As for minor leg injuries, a relationship with APC resistance has not been studied and does not seem likely. Therefore, we propose a different mechanism here: because of the nature of the injuries, it may be that a thrombus develops locally rather than that systemic activation of the clotting system occurs. Ankle sprains, for example, will probably lead to local thrombus formation because of tissue damage and restrained mobility. In most cases, however, patients with this kind of injuries are not immobilized for a long period of time and tend to recover quickly. These patients could therefore be less likely to develop a PE than a DVT.
A higher risk of PE than of DVT was defined as RR(PE)/RR(DVT) ≥ 2. We found that COPD and sickle cell disease fulfilled this criterion. Indeed, they were found to have no or little effect on DVT.
Sickle cell trait gave a 4-fold increased risk of PE, whereas for DVT the risk was not increased at all (OR = 1.1; 95% CI, 0.7-1.9).13 Sickle-shaped cells have a tendency to form clots; and once formed, these clots are easily trapped in the microcirculation of the brain or in the smaller pulmonary arteries. This may occur in an acute chest syndrome, where hypoxia further enhances coagulation. This local chronic inflammatory reaction may predispose for PE. Of note, as sickle cell trait has a high prevalence in blacks, this could in part explain the higher risk of PE than DVT in blacks compared with whites.13
For COPD, there were 2 studies that both showed effect estimates that were higher for PE than for DVT (L.E.F., A.v.H.V., K. W. van Kralingen, S.C.C., and F.R.R., Chronic obstructive pulmonary disease and pulmonary embolism, manuscript in preparation).48 As with sickle cell disease, an explanation may be sought in the inflammatory component of COPD and asthma, the effect of which on the coagulation system has been summarized in a recent review.75 This hypothesis is strengthened by a recent finding in the MEGA study: that pneumonia, as an acute inflammatory process in the lungs, increased the risk of PE with an OR of 7.9 (6.1-10.3). For DVT alone, the OR was 3.0 (95% CI, 2.2-4.0; Ribeiro et al76 ). Pneumonia has been shown to be a risk factor for VT in a study on respiratory and urinary tract infections performed in a general practioners' patient population in the United Kingdom. However, no separate risk estimates were given for DVT and PE.77
Although the mechanisms we propose are theoretically plausible, there may be other biologic explanations for the associations we found of which we are not aware. Other explanations are related to bias or misclassification and are discussed in “Limitations.”
Limitations
First, relatively few studies (∼ 10%) of our initial literature search yielded relevant information to answer our research question. Of the studies we included, we depended on their methodology that could have had limitations in terms of possible bias and confounding. Second, there may have been misclassification in the DVT and PE patient groups, as one of the 2 VT presentations may sometimes coincide symptomless with the other. Up to 40% of DVT patients have been shown to have a concomitant silent PE.78 In addition, in the overview of current literature, we would have preferred to include only studies with proven isolated PE, and not all studies provided this. Nevertheless, through such misclassification, the contrast we found between PE and DVT will at most have been diluted. Therefore, differences that we have found are likely to have been larger had selection criteria been followed more strictly. Third, oral contraceptives were shown to be a stronger risk factor for DVT than for PE in 2 studies (criterion met).32,33 However, the WHO study results were presented separately for Europe and other continents (Africa, Asia, and Latin America), and the effect estimates for Europe were not in the same direction as for the other regions.33 As ethnicity has a strong impact on risks for PE and DVT, we believe the scarce results on non-Europeans are difficult to interpret.
Finally, we found several pulmonary conditions that conferred a higher risk for PE than DVT. COPD patients may be more likely to undergo a CT scan of the thorax than an ultrasonography of the legs because of their clinical complaints, thereby increasing the probability of finding a PE. Likewise, patients with obesity may have other conditions that lead to more frequent testing of the legs (edema). Therefore, we cannot exclude that part of the effects we found may be explained by an increased frequency of imaging of certain patients.
Two different sides of the VT spectrum
We found several risk factors for VT that have a differential effect on PE and on DVT. Therefore, the etiology of PE and DVT is apparently not always the same, and we suggest that PE and DVT are 2 different sides of the VT spectrum. A difference is also apparent in the pattern of recurrent VT events. If DVT and PE are merely different expressions of the same disease, the anatomic location of recurrence would be expected to be random (ie, independent of the location of the first event). However, after a PE, patients are more likely to develop a recurrent PE rather than a DVT, whereas patients with a DVT more often develop recurrent DVT.15,79,80 Another explanation for this finding is that patients may be more alert to recurrent symptoms of chest pain or leg pain that resemble the symptoms of their first VT event. In addition, PE can lead to a poor cardiopulmonary reserve, which makes a patient more likely to experience PE symptoms again.
In conclusion, in this overview of the literature, enriched with data from the MEGA study, we assessed whether differential effects for DVT and PE could be confirmed for other common VT risk factors than FVL in an effort to broaden and understand the “FVL paradox.” We found that the paradox could indeed be expanded by several risk factors with a higher risk for DVT than for PE. The differential effect of most of these risk factors (oral contraceptive use, pregnancy, puerperium, and obesity) can be explained through increased APC resistance. In addition, minor leg injuries gave a higher risk of DVT than of PE, possibly because of the local effect of trauma. Pulmonary inflammatory diseases, such as pneumonia and COPD, as well as sickle cell disease, increased the risk of PE but posed little risk of DVT. Therefore, we propose that PE and DVT should not always simply be considered as 2 manifestations of the same disease. These findings are not purely academic and may have implications for prophylaxis regimens because the balance of preventing thrombosis at the cost of bleeding should depend on whether the prevented events are DVTs or PEs. They therefore encourage unraveling the different mechanisms in further studies.
This online version of this article contains a data supplement.
Acknowledgments
The authors thank J. W. Schoones (Walaeus Library, Leiden University Medical Center, Leiden, The Netherlands) for his assistance with the literature search and J. T. Padding (Department of Chemical Engineering and Chemistry, Eindhoven University of Technology, Eindhoven, The Netherlands) for his help with the criteria of difference in disease incidence.
Authorship
Contribution: K.v.L. collected and analyzed the data and drafted the manuscript; L.E.F. collected and analyzed the data and reviewed the manuscript; A.v.H.V. and F.R.R. reviewed the manuscript; and S.C.C. designed the research and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Suzanne C. Cannegieter, Department of Clinical Epidemiology, C7-P, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail: s.c.cannegieter@lumc.nl.