Abstract
S0515 was a phase 2 trial to determine whether the addition of bevacizumab to cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) plus rituximab (R-CHOP) would improve progression-free survival (PFS) without adding significant toxicity in patients with newly diagnosed advanced diffuse large B-cell lymphoma. A total of 73 patients were enrolled. For the 64 eligible patients, median age was 68 years, and 60% had International Prognostic Index scores more than or equal to 3. The observed 1- and 2-year PFS estimates were 77% and 69%, respectively. These PFS estimates were not statistically different from the expected PFS for this population if treated with R-CHOP alone. Grade 3 or higher toxicities were observed in 81% of patients, including 2 grade 5 events. The majority of serious toxicities were hematologic but also included 5 patients with gastrointestinal perforations, 4 patients with thrombotic events, and 11 patients who developed grade 2 or 3 left ventricular dysfunction. Higher baseline urine VEGF and plasma VCAM levels correlated with worse PFS and overall survival. In conclusion, the addition of bevacizumab to R-CHOP chemotherapy was not promising in terms of PFS and resulted in increased serious toxicities, especially cardiac and gastrointestinal perforations. This study is registered at www.clinicaltrials.gov as #NCT00121199.
Introduction
Angiogenesis plays a critical role in the growth and metastasis of multiple solid and hematologic malignancies. Elevated levels of plasma angiogenic factors, including VEGF and VCAM, are associated with poor overall survival (OS) and progression-free survival (PFS) in clinical trials and VEGF, and its receptors are frequently expressed in lymphoma specimens by immunohistochemistry or gene expression profiling.1-6 Resistance to chemotherapy has also been correlated with high levels of VEGF expression in non-Hodgkin lymphoma (NHL) and in xenograft models.7-10 Treatment with anti-VEGF therapy plus rituximab or chemotherapy yielded superior antitumor responses compared with either therapy alone.9
In the SWOG 0108 trial of single-agent bevacizumab therapy in patients with relapsed, aggressive NHL, patients with elevated levels of urine VEGF and plasma VCAM had a worse OS and PFS compared with patients with lower levels of these angiogenic factors.1 On immunohistochemical analysis, more than 60% of specimens from diffuse large B-cell lymphoma (DLBCL) expressed VEGF and its receptors, VEGFR-1 and VEGFR-2.1 These data suggested that combining standard antilymphoma therapy with VEGF-targeted agents may provide superior efficacy in the treatment of patients with DLBCL. Bevacizumab, a monoclonal anti-VEGF antibody, has been the most extensively studied antiangiogenic agent and has shown antitumor activity in a number of tumor types, especially when combined with standard chemotherapy regimens.11 As a monoclonal antibody, bevacizumab also has several advantages over tyrosine kinase inhibitors that also target the VEGF pathway, including improved specificity for VEGF, a well-defined toxicity profile, defined pharmacokinetics, and a long half-life that allows for synchronous dosing with cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) plus rituximab (R-CHOP), the current standard for treating newly diagnosed DLBCL. SWOG 0515 was initiated to determine the feasibility, safety, and efficacy of combining bevacizumab to standard therapy with R-CHOP in patients with newly diagnosed, advanced DLBCL.
Methods
Patient selection
Patients with previously untreated stage 3, stage 4, or bulky stage 2 diffuse large B-cell NHL (DLBCL) positive for CD20 were eligible. Enrollment was initially limited to patients more than or equal to 65 year of age or if 60 to 64 years with an age-adjusted International Prognostic Index (IPI) score of 0 or 1. The protocol was subsequently amended to include all patients more than or equal to 18 years of age with CD20+ DLBCL once a competing SWOG protocol was closed to accrual. Additional eligibility criteria included a Zubrod performance status of more than or equal to 2, left ventricular ejection fraction (LVEF) more than or equal to 45% by multiple uptake gated acquisition scan or ECHO, neutrophil count more than 1000/μL, platelet count more than 100 000/μL, serum creatinine less than 2 × institutional upper limit of normal, and urine protein/creatinine ratio less than 1.0. Prior chemotherapy, radiation therapy, or antibody-based therapy for lymphoma was not permitted. Patients with a history of indolent lymphoma, CNS involvement, HIV or hepatitis B infection, solid tumor transplant, uncontrolled hypertension, pregnant or nursing, history of arterial thrombosis or bleeding diathesis/coagulopathy, history of abdominal fistula, abscess, or gastrointestinal (GI) perforation, or major surgical procedure or traumatic injury within 28 days were also excluded. In addition, patients with clinically significant peripheral vascular disease, nonhealing wounds, ulcers, or bone fractures, or requiring continuous supplemental oxygen therapy or chronic oral or parenteral anticoagulants were ineligible.
All patients were informed on the investigational nature of the study and provided written informed consent in accordance with institutional and federal guidelines as well as the Declaration of Helsinki. This study was approved by the Institutional Review Board of the University of Arizona Cancer Center and all participating sites.
Study design
S0515 was a nonrandomized phase 2 clinical trial conducted through SWOG. The study was activated in June 2005 and closed to accrual in September 2008. All patients on study received standard-dose CHOP (cyclophosphamide 750 mg/m2, vincristine 1.4 mg/m2 up to a maximum dose of 2 mg, and adriamycin 50 mg/m2 on day 1, prednisone 100 mg on days 1-5), and rituximab 375 mg/m2 on day 1, and bevacizumab 15 mg/kg on day 1. All therapy was given intravenously, except for prednisone, which was administered orally. Cycles were administered every 21 days for a maximum of 8 cycles. Rituximab and bevacizumab were administered before the chemotherapy; and for cycle 1 only, the therapy could be divided over several days with bevacizumab given on day 0, CHOP on day 1, and rituximab on day 2.
Supportive measures, including administration of allopurinol and the use of erythropoietin or granulocyte growth factors, prophylactic antibodies, and aspirin up to 325 mg/day, were permitted. Blood pressure was assessed weekly during the first cycle and before administration of bevacizumab. Cardiac function was monitored at baseline and after cycle 4 and cycle 8 or the completion of therapy. Abnormal multiple uptake gated acquisition scans were confirmed by echocardiograms. Bevacizumab therapy was discontinued for a fall in the LVEF less than 45% associated with abnormal wall motion.
Patients were restaged after cycle 4 and cycle 8, and patients with disease progression were withdrawn from study. After completion of protocol treatment, patients underwent restaging at least once every 6 months for 2 years, and then annually for a maximum of 5 years. Physicians were required to use the same imaging modalities for staging patients throughout the study. The majority of patients were initially staged by chest, abdominal, and pelvic CT scans with a minority staged by PET/CT scans. A bone marrow aspirate and biopsy were performed on all patients at diagnosis, and patients with involved bone marrow were required to have a repeat examination documenting response. Response criteria were per the proposed International Workshop criteria.12 Urine and blood for biomarker studies were obtained at baseline and before cycles 4 and 8 of therapy.
Biomarker studies
Patient samples (urine, plasma, and whole blood) were collected for analysis of urine VEGF, plasma VCAM and VEGF, and circulating endothelial cells (CECs). Plasma VCAM, VEGF, and urinary VEGF levels were quantified by ELISA kits (R&D Systems). CECs were measured by flow cytometry (FACScan, Becton Dickinson) on whole blood using a red blood cell lysis method previously described.1
Tissue diagnosis (needle aspirations not allowed) of DLBCL was confirmed by central pathology review (L.M.R.). Immunohistochemical analysis was performed on formalin-fixed, paraffin-embedded tissue microarrays. Slides were stained for expression of VEGF (Santa Cruz Biotechnology) and VEGFR-1 (Thermo Fisher Scientific) using rabbit polyclonal antibodies and for VEGFR-2 (Cell Signaling Technology) using rabbit monoclonal antibodies. All reactions were performed using an automated immunostainer (Ventana Discovery XT autostainer, Ventana Medical Systems) using a previously published method.13 The degree of expression in endothelial and lymphoma cells was determined at 400× magnification as 0 (no expression), 1+ (faint), 2+ (moderate), or 3+ (strong) from 2 separate 1-mm tissue cores by a pathologist (B.F.) with the higher score being recorded. Controversial cases were reviewed by a second pathologist (L.M.R.).
Statistical considerations
The primary end point of this study was the 1-year PFS rate. Seventy eligible patients were planned to be enrolled. The statistical design stipulated that 70 patients accrued over 18 months with 12 months of additional follow-up would be sufficient to estimate the 1-year PFS to within ± 0.12. Given that this was a single-arm study, the design also specified an adjustment for important prognostic groups. Historical R-CHOP data show that 1-year PFS is approximately 70% for patients older than 60 years and for younger patients (≤ 60 years old) with high IPI scores (≥ 2). In addition, Pfreundschuh et al showed that 1-year PFS was approximately 90% for patients 60 years of age or younger with low IPI scores (0 or 1).14 Given these historical data and assuming that, for example, 10% of patients would be in the younger patient group (≤ 60 years old) with low IPI, the weighted 1-year PFS for R-CHOP would be 72%. Under these assumptions, an estimated 1-year PFS of 81% or greater (based on the 95% upper bound on the confidence interval using a one-arm survival design) would be sufficient to warrant further investigation.15 However, the target value will be adjusted based on the observed frequency of prognostic groups. Secondary end points include OS and response. Seventy patients are also sufficient to estimate the probability of any particular toxicity to within ± 0.12. Any adverse event with at least a 5% probability will be seen at least once (97% chance). Assuming an additional 24 months of follow-up from completion of accrual, 70 patients is sufficient to estimate the 2-year PFS to ± 0.12. Survival was estimated according to the method of Kaplan and Meier.16 Analyses of survival differences by prognostic factors were performed using Cox regression.17 Groups were divided into low and high by the median level of the biomarker analyzed (urine and plasma VEGF, plasma VCAM, and CEC). The median level was chosen as the cut-point for these analyses as it provides for the most power assuming a continuous effect across the spectrum of values for a given biomarker, for ease of interpretation, and to avoid testing multiple cut-points. Differences in biomarker values between baseline and follow-up were tested using a paired t test.
Results
Patient characteristics
A total of 73 patients were registered to the study. Nine patients were ineligible: 7 secondary to ineligible histologies (predominantly because of follicular component) and 2 with insufficient baseline information. Baseline characteristics of the 64 eligible patients are summarized in Table 1. The median age was 68 years, with 77% of patients older than 60 years and 6 patients (9%) older than 80 years. The majority of patients had stage 3 or 4 disease, and 79% of patients had intermediate-risk IPI scores of 2 or 3. Forty-five (or 70%) of patients completed all 8 cycles of protocol therapy. Reasons for not completing protocol therapy included adverse events in 11 (17%) patients, death in 3 (5%), disease progression in 1 patient, patient decision in 1 patient, or other (non–protocol-specified) in 3 patients. The median follow-up for patients still alive is 3.5 years (maximum, 5.8 years).
Clinical efficacy
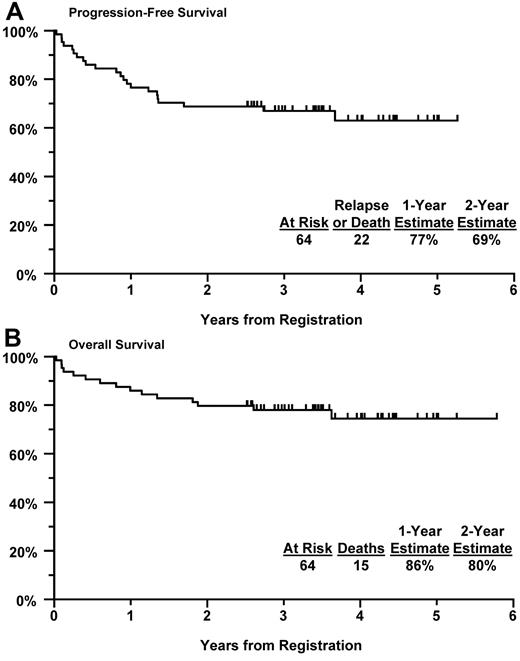
The 1-year PFS estimate was 77% (95% CI, 66%-87%) with a 1-year OS estimate at 86% (95% CI, 77%-94%; Figure 1). The 2-year PFS and OS estimates were 69% (95% CI, 57%-80%) and 80% (95% CI, 70%-90%), respectively. To compare these results with those expected with treatment with R-CHOP alone, the 1-year target PFS for this population with R-CHOP alone was estimated based on the following assumptions: (1) for patients older than 60 years or those 60 years of age or younger with IPI more than or equal to 2 (representing 94% of the treated population for this study), the PFS is 70%; and (2) for patients 60 years of age or younger with IPI 0 or 1 (representing 6% of the treated population), the PFS is 90%. Based on the observed frequencies, the weighted target estimate of 1-year PFS would be 71% with R-CHOP alone; given this, a 1-year PFS of 80% or greater would warrant further investigation of this regimen. Although the observed 1-year PFS of 77% exceeded the estimated rate of 71% with R-CHOP alone, it did not achieve the adjusted target 1-year PFS estimate of more than or equal to 80%. Using a similar approach, the expected 2-year PFS with R-CHOP alone for this population was estimated at 62%, with a rate of more than or equal to 73% indicating that further investigation is warranted. Our observed 2-year PFS trended higher at 69% but also did not achieve the prespecified level of 73%.
Kaplan-Meier curves. (A) PFS in all eligible patients. (B) OS in all eligible patients.
Kaplan-Meier curves. (A) PFS in all eligible patients. (B) OS in all eligible patients.
The best observed response rate was a complete response in 44% of patients (22 confirmed and 6 unconfirmed) and a partial response in 33% of patients (20 confirmed and 1 unconfirmed) for an overall response rate of 77%. Two patients had progressive disease, 3 patients died before evaluation, 1 patient had stable disease as their best response, and 9 patients had inadequate assessments.
Toxicities
Sixty-three of the 64 eligible patients were evaluated for toxicity (no toxicity data were reported for 1 patient). A summary of grade 3 (serious) and 4 (life-threatening) toxicities is shown in Table 2. Overall, 81% of patients had grade 3 or worse toxicity, including grade 3 or 4 in 78% and grade 5 in 2 patients (3%), who experienced sudden deaths deemed possibly related to treatment. The majority of patients (62%) experienced at least serious hematologic toxicity, including febrile neutropenia in 11 patients (8 grade 3 and 3 grade 4), despite growth factor support being strongly recommended. Serious GI toxicity was reported in 14 patients (22%), including 5 cases of GI perforation. One patient had recurrent episodes of GI perforation requiring hospitalization despite discontinuation of bevacizumab with subsequent R-CHOP therapy and was probably caused by tumor necrosis involving bowel wall. All patients discontinued bevacizumab after the event and 3 required bowel resection. One patient died of complications of the perforation, including sepsis with respiratory failure. The thrombotic events included 2 pulmonary emboli (1 incidental) and 2 lower extremity deep vein thromboses.
The rate of grade 3 or 4 adverse events was only somewhat higher in older patients (79% vs 73%), whereas all 6 patients older than 80 years had at least serious toxicity (100%). None of the patients older than 80 years completed 8 cycles of protocol therapy.
Among the 9 ineligible patients, 6 (67%) had grade 3 or 4 toxicity, most of which was hematologic (n = 5), including 2 additional cases of grade 3 febrile neutropenia.
Because of the concern for possible increased cardiac toxicity when combining bevacizumab with anthracycline therapy, additional cardiac monitoring was instituted, including evaluation of left ventricular function (LVEF) at baseline, and after cycle 4 and 8 of therapy. A grade 2 or greater drop in LVEF was observed in 11 (17%) evaluable patients, including 7 patients with grade 2 (LVEF, 40%-50%) and 4 patients with grade 3 (LVEF, 20%-40%) LVEF dysfunction. The average and median age of patients developing cardiac dysfunction was 67.7 years. Cardiac arrhythmia, including atrial fibrillation or sustained ventricular tachycardia, was observed in an additional 3 patients.
Additional bevacizumab-associated toxicities included grade 3 (4 patients) and grade 2 (2 patients) hypertension. An additional 22% (n = 14) of patients experienced epistaxis, with the majority being grade 1 (11 patients) though 2 grade 3 events were noted. Grade 3 or higher proteinuria was not observed with only one grade 1 adverse event reported.
To assess the relative frequency of cardiac and GI toxicity for this regimen, we compared rates from this study to a contemporary SWOG pilot study (S0433) of R-CHOP + I-131 tositumomab in the same patient population.18 The rate of serious or worse (grade 3 or 4) cardiovascular toxicity on S0433 was 7% (6 of 84, including 2 cardiac deaths) and on S0515 was 16% (10 of 63), but this difference was not statistically significant (P = .16). However, the rate of serious or worse (grade 3 or 4) GI toxicity on S0433 was 6% (5 of 84) with no GI perforations reported and on S0515 was 22% (14 of 63), representing a statistically significant difference (P = .008). Given difficulties in comparing toxicities between 2 groups of nonrandomized patients, this analysis nonetheless suggests trends toward distinctive toxicities related to bevacizumab.
Biomarker results
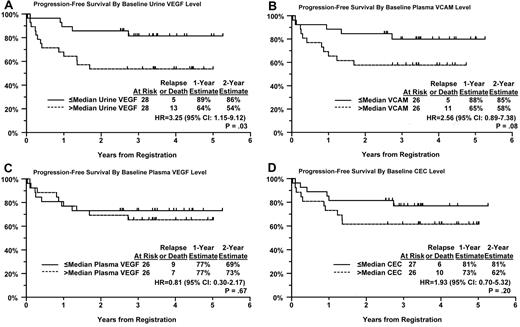
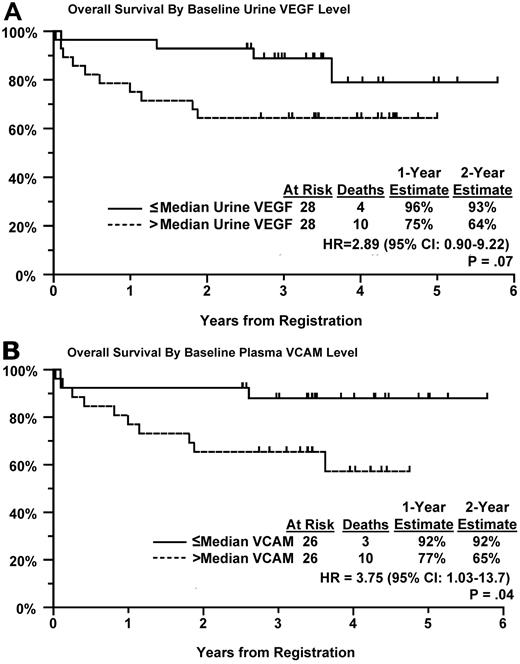
In our previous trial, S0108, in which patients with relapsed, aggressive NHL was treated with single-agent bevacizumab, patients with elevated baseline levels of urine VEGF, and plasma VCAM were found to have inferior PFS and OS compared with patients with lower levels.1 We performed a similar analysis in patients from this trial with newly diagnosed DLBCL. Baseline values were available for more than 80% of eligible patients, approximately 50% of patients had both baseline and repeat samples at follow-up. Patients were divided into 2 equal sized groups based on the level of angiogenic biomarker above or below the median. Patients with higher levels of urine VEGF and plasma VCAM had significantly or marginally significantly worse PFS (P = .03 and P = .08, respectively; Figure 2A-B) and OS (P = .07 and P = .04, respectively; Figure 3A-B) compared with patients with values below the median level (Table 3). OS was estimated at greater than 90% at 2 years in patients who had lower median levels of urine VEGF or plasma VCAM at baseline (Figure 3A-B). Urine VEGF levels were highly correlated with plasma VCAM levels (P = .006). However, elevated VCAM or urine VEGF levels were not associated with higher IPI scores (P = .38 and .26, respectively), suggesting that these angiogenic biomarkers have prognostic significance irrespective of the IPI. Indeed, after adjustment for their IPI score, both urine VEGF and plasma VCAM levels still correlated with PFS (P = .04 and P = .08, respectively) and OS (P = .11 and P = .05, respectively) in a multivariate model.
PFS by Kaplan-Meier curves for patients. Based on their baseline level of (A) urine VEGF, (B) plasma VCAM, (C) plasma VEGF, and (D) CECs.
PFS by Kaplan-Meier curves for patients. Based on their baseline level of (A) urine VEGF, (B) plasma VCAM, (C) plasma VEGF, and (D) CECs.
OS by Kaplan-Meier curves for patients. Based on their baseline level of (A) urine VEGF and (B) plasma VCAM.
OS by Kaplan-Meier curves for patients. Based on their baseline level of (A) urine VEGF and (B) plasma VCAM.
Plasma VEGF was also measured (Figure 2C), but a similar relationship was not observed despite requesting rapid centrifugation and freezing of the samples after phlebotomy. These results may be secondary to the technical difficulties in obtaining accurate plasma values of VEGF.19,20 Our inability to find a correlation between plasma and urine levels of VEGF also support the observation of others that plasma VEGF levels are more clearly related to platelet-derived rather than circulating or tumor-derived VEGF.19,20 CECs were also quantified by flow cytometry. Patients with higher baseline levels trended to have worse PFS, but this did not reach statistically significance (Figure 2D; P = .20). There was a wide range of values seen for all the angiogenic biomarkers tested. Baseline and longitudinal values (cycles 4 and 8) are shown in Table 4, where sample sizes are limited to patients with 3 values obtained. Differences between baseline and follow-up biomarker distributions were evident for plasma VEGF (cycles 4 and 8) and plasma VCAM (cycle 4 only). A significant decrease in urine VEGF was not observed though the values trended down at cycle 4.
Immunohistochemistry results
Forty eligible patients provided acceptable tissue for analysis of VEGF and VEGF receptor expression from their diagnostic biopsies. VEGF expression was observed in 98% of these lymphoma specimens and was moderate or strongly expressed (≥ 2+) in 80% (Table 5). Endothelial cells also expressed VEGF in 90% of cases, although typically at less intensity than the lymphoma cells. VEGFR-1 was expressed in 93% of lymphoma cells and all endothelial cells, whereas VEGFR-2 was preferentially expressed on endothelial cells (95%) compared with lymphoma cells (15%).
Discussion
The results of this phase 2 study confirm the relevance of angiogenesis in DLBCL. Almost all lymphoma cells expressed VEGF, with more than 80% expressing moderate to strong levels by immunohistochemistry. VEGF receptor 1 and 2 was also expressed at high levels in lymphoma cells and/or tumor-associated endothelial cells. Plasma VCAM and urine VEGF levels predicted PFS and OS in the patients. Thus, the clinical results obtained from combining bevacizumab with standard R-CHOP are particularly disappointing. Patients with elevated angiogenic markers responded poorly to R-CHOP therapy despite the addition of bevacizumab. Thus, specifically targeting the VEGF pathway did not improve chemotherapy or immunotherapy responsiveness in patients with elevated baseline levels of VEGF or VCAM.
Our angiogenic biomarker results are particularly interesting as they confirm our prior trial results from S0108 that urine VEGF and plasma VCAM levels are prognostic, and patients with high levels of these angiogenic factors continue to perform poorly, even with antiangiogenic therapy, such as bevacizumab.1 Of note, plasma VEGF levels were not prognostic in our study. The utility of plasma or serum VEGF levels as a prognostic or predictive biomarker in oncology has been inconsistent in the literature.21-23 This is probably secondary to the difficulties in accurately measuring tumor released or associated VEGF as recent data suggest that plasma or serum measurements of VEGF are highly dependent on platelet count and phlebotomy technique.19,20 Thus, our results with urine VEGF and plasma VCAM may be more relevant clinically, precisely because we have chosen to study a more robust, and therefore predictive, marker of in vivo VEGF effects. In particular, because VCAM expression is directly related to VEGF stimulation on endothelial cells and as a biomarker is relatively stable in plasma and easily quantified using sensitive and specific ELISA kits, VCAM may be the best currently available biomarker for measuring in vivo activity of the VEGF pathway.
CEC levels were also measured as a potential biomarker for bevacizumab activity.24,25 Others, including SWOG's prior work in S0108, suggested that a fall in CEC levels predicted response.1,22 In S0515, no such trend was observed; however, the recommended use of G-CSF and aggressive chemotherapy may have led to increased mobilization of bone marrow-derived endothelial precursor cells that prevented measuring the effect of bevacizumab on CECs.26,27 Elevated levels of CEC are thought to reflect increased vascular remodeling and turnover consistent with the trend observed of worse PFS in patients with elevated baseline CEC levels.28
The increased toxicities associated with combining VEGF-targeted therapy to standard R-CHOP should also be noted, especially if further trials testing other VEGF-targeted agents in combination with chemotherapy are proposed in this population. An increased incidence of cardiac events with decreases in left ventricular ejection fractions have been noted in other bevacizumab trials and particularly in trials in which anthracyclines have been combined with bevacizumab.29-32 There are data from our trial as well as the literature that the decrease in LVEF may be reversible though the percentage who completely recover function, and the risk factors for left ventricular dysfunction are currently unknown. In our trial, the median age in patients with grade 2 or higher LVEF dysfunction was 68 years, the same as in our entire study group. Cardiac toxicity was observed in patients as young as 47 years. The pathogenesis of bevacizumab-induced cardiotoxicity is currently unknown. Increased VEGF levels are associated with myocardial ischemia and increased coronary collaterals in animal models.33,34 Increased hypertension may also lead to increased stress on the heart where VEGF plays a pivotal role in maintaining myocardial capillary density. Inhibitors of VEGF can inhibit the compensatory hypertrophy seen in response to stress and thus lead to more rapid transition to heart failure.35 In our trial, any grade hypertension was observed in 7 (11%) patients, all of whom also developed grade 3 or 4 cardiac toxicity. However, 17 of our patients with grade 3 or 4 cardiac toxicity did not have hypertension. In our study, hypertension was not observed more commonly compared with other trials of bevacizumab.30 Other VEGF inhibitors, including sunitinib and sorafenib, have also been associated with increased cardiotoxicity in clinical trials.36-38
GI perforation was also seen more commonly than observed in trials of R-CHOP alone.18,39 Interestingly, 4 of the 5 patients who developed GI perforations developed symptoms after their first cycle of therapy, suggesting that GI involvement by DLBCL is frequently asymptomatic and undiagnosed. A higher incidence of GI perforation is observed with bevaczumab when administered to cancers known to involve the GI tract and in colorectal cancer when the primary is unresected.40,41 If a subset of patients that benefit from anti-VEGF therapy could be ultimately identified, these findings suggest that it may be safer to initiate bevacizumab therapy after the first or second cycle of R-CHOP to decrease the incidence of GI perforation. However, the increase in both urine VEGF levels and, in particular, plasma VCAM levels before cycle 8 of therapy, suggest that there may be feedback loops that can override VEGF-targeted therapies and lead to activation of alternative proangiogenic pathways with the resumption of angiogenesis. Thus resistance to bevacizumab may be an additional issue, particularly if the antiangiogenic therapy is directed against a single pathway (ie, VEGF) or for a short time frame.
The multitargeted VEGF receptor kinase inhibitor, sunitinib, has also been tested as a single agent in DLBCL with similar poor results.42 The study was closed after the first stage secondary to increased toxicity (predominantly hematologic), no evidence of clinical activity, and no significant change in their pharmacodynamic marker of CECs.42
In the first publication of bevacizumab plus R-CHOP in 13 patients with DLBCL, the 1-year PFS compared similarly with our result at 77%.43 Most grade 3 and 4 toxicities were hematologic as observed in our study, but GI perforations and cardiotoxicity were not observed in this much smaller and younger cohort (median age, 49 years). Our study also supports the continued use of adequately powered phase 2 trials in predicting important toxicities and efficacy before initiating large and expensive phase 3 trials. Before the completion of this trial, Roche did initiate a large, international phase 3 trial randomizing patients with newly diagnosed DLBCL to R-CHOP versus R-CHOP plus bevacizumab. An independent Data and Safety Monitoring Board recommended closure of the trial after the first 720 patients were randomized secondary to increased toxicity, including higher rates of LVEF reductions and an unlikely benefit-risk assessment in the R-CHOP plus bevacizumab arm.42 Hundreds of patients would have been spared the increased toxicities and risks associated with this regimen if the launch of the phase 3 trial awaited the results of S0515.
The encouraging results of the 2-year PFS more than 85% and OS more than 90% in patients with lower levels of baseline angiogenic markers is notable and, if confirmed in larger trials, suggests that the population to target for new and novel therapies is those patients with high baseline angiogenic markers. The poor prognosis of patients with elevated baseline angiogenic levels, despite being treated with VGEF-targeted therapy, suggests that other growth pathways may also be up-regulated in these aggressive lymphomas. The constructed tissue microarrays from this study can be used to further classify the specimens into germinal center B cell-like or activated B cell-like phenotypes and suggest other pathways for targeting in future studies.
In conclusion, the addition of bevacizumab to standard R-CHOP is not promising with respect to PFS or OS in patients with newly diagnosed DLBCL, especially in lymphomas that overexpress angiogenic biomarkers. In addition, this regimen is associated with increased life-threatening toxicities, including GI perforation and cardiac dysfunction, which suggest that further evaluation of this combination in unselected patients with DLBCL is unjustified.
Presented in part at the 2010 Annual Meeting of the American Society of Hematology, Orlando, FL, December 6, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the following Public Health Service Cooperative Agreement grant numbers awarded by the National Cancer Institute, Department of Health and Human Services: CA32102, CA38926, CA58861, CA45807, CA35431, CA67575, CA63848, CA13612, CA76429, CA45377, CA35261, CA45808, CA76447, CA35176, CA45560, CA45450, CA46113, CA37981, CA42777, CA63844, and CA12644.
National Institutes of Health
Authorship
Contribution: A.T.S. and T.P.M. designed research, interpreted data, and wrote the manuscript; J.M.U. designed research, interpreted data, performed statistical analysis, and wrote the manuscript; L.M.R. and B.F. interpreted data, performed research, and wrote the manuscript; M.L. designed research, interpreted data, and performed statistical analysis; M.I. performed research and interpreted data; M.J.G. collected data; and R.I.F. designed research and interpreted data.
Conflict-of-interest disclosure: A.T.S. received honoraria for consulting with Genentech. R.I.F. was a consultant and received speaking honorarium from Genentech/Roche. The remaining authors declare no competing financial interests.
Correspondence: Alison T. Stopeck, Arizona Cancer Center, PO Box 245024, 1515 N Campbell Ave, Tucson, AZ 85724-5024; e-mail: astopeck@azcc.arizona.edu.