Abstract
Transplantation of ex vivo expanded human umbilical cord blood cells (hCB) only partially enhances the hematopoietic recovery after myelosuppressive therapy. Incubation of hCB with optimal combinations of cytokines and niche cells, such as endothelial cells (ECs), could augment the efficiency of hCB expansion. We have devised an approach to cultivate primary human ECs (hECs) in serum-free culture conditions. We demonstrate that coculture of CD34+ hCB in direct cellular contact with hECs and minimal concentrations of thrombopoietin/Kit-ligand/Flt3-ligand resulted in a 400-fold expansion of total hematopoietic cells, 150-fold expansion of CD45+CD34+ progenitor cells, and 23-fold expansion of CD45+ Lin−CD34hi+CD45RA−CD49f+ stem and progenitor cells over a 12-day period. Compared with cytokines alone, coculture of hCB with hECs permitted greater expansion of cells capable of multilineage engraftment and serial transplantation, hallmarks of long-term repopulating hematopoietic stem cells. Therefore, hECs establish a cellular platform for expansion of hematopoietic stem and progenitor cells and treatment of hematologic disorders.
Introduction
Human umbilical cord blood (hCB) is an attractive source of donor cells for allogeneic transplantation.1 Nonetheless, the limited number of engraftable hematopoietic stem and progenitor cells (HSPCs) in hCB is associated with prolonged cytopenias after transplantation and even graft failure in lethally ablated recipients. To circumvent this hurdle, hCB has been expanded with a combination of cytokines,2 prostaglandin E2,3 angiopoietins,4 Notch ligands, and HoxB4.5,6 Coculture of HSPCs with human stromal7 and endothelial cells (ECs)8,9 has also been shown to augment expansion of HSPCs.
However, the role of human ECs (hECs) in the homeostasis of human HSPCs has been hampered by a lack of physiologic models. In the absence of serum and angiogenic factors (VEGF-A/FGF-2/IGFs/EGF), primary hECs undergo apoptosis. We have established a method to maintain primary hECs by introducing the E4ORF1 gene of adenoviruses (E4+ECs) to enable their long-term survival in serum-free/growth factor-free conditions.10 We demonstrate that E4+ECs support long-term expansion of engraftable human CD45+ hematopoietic cells, CD45+CD34+ progenitors, and CD45+Lin−CD34hi+CD45RA−CD49f+ HSPCs.
Methods
In vitro cultures
E4+ECs were generated by introducing a lentiviral vector expressing E4ORF1 gene into primary human umbilical vein ECs (hECs).10 A total of 5 × 104 hCB cells isolated with anti-CD34 microbeads (Miltenyi Biotec) were cocultured in serum-free X-Vivo20 (n = 10) or StemSpan (n = 4) supplemented with 50 ng/mL of SCF + thrombopoietin (TPO) + Fms-like tyrosine kinase 3 ligand (Flt3L; STF media), with E4+ECs (+ cytokines) or without feeder cells (cytokines alone). Cumulative expansions of CD34+ cells were analyzed at day 12 of coculture. When analyzing the expanded hCB cells, we depleted E4+ECs cells by positive selection of hematopoietic cells with anti-CD45 microbeads. Expanded cells were analyzed by FACS and/or in vivo transplantation assays. These experiments were also repeated using high-dose cytokines (300 ng/mL SCF, 300 ng/mL Flt3L, 100 ng/mL TPO, 100 ng/mL IL-6, and 10 ng/mL IL-3).
Flow cytometry
FACS analyses were performed using a BD Bioscience LSRII/SORP and the following antibodies: CD45-APC, BV421-(HI30), CD34-PECy7-(581), CD38-v450 or APC-(HB7), CD45RA-APC-Cy7-(HI100), and CD49f-PE-(GoH3) for HSPCs; CD33-PE or PE-Cy7-(P67.6), CD14-PECy7 or APC-(M5E2), and CD15-FITC or PE-(HI98) for monocytes; CD51/61-FITC or PE-(23C6), CD42b-APC-(HIP1), CD41a-FITC, or PE-Cy7-(HIP8) for megakaryocytes; CD19-FITC-(HIB19) and IgM-FITC-(G20-127) for B cells; and CD4-PE-Cy7-(SK3) for T cells. For in vivo transplantation assays, we gated out mouse cells using CD45 and Ter119.
In vivo transplantation
All mice experiments were approved by the Weill Cornell Medical College Institutional Review Board. Ten-week-old NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice were sublethally irradiated (325 cGy) and transplanted with 1 × 106 CD45+ expanded hCB cells. Engraftment was assessed at 3 weeks (peripheral blood); and at 9 weeks, the femurs/tibias/spleens were removed and numbers and types of human HSPCs were determined. We performed secondary transplantations by isolating human BM cells from femurs/tibias and cultured in STF media + 20 ng/mL of IL-6 for 48 hours and then transplanted into sublethally irradiated secondary NSG recipients. The frequency of SCID-repopulating cells was determined by limiting dilution assays (L-Calc Version 1.1 software; StemCell Technologies) and transplanting various doses of expanded CD34+-enriched cells.
Statistical analysis
A P value less than .05 (Student t test) was considered significant. Results are expressed as mean plus or minus SD.
Results and discussion
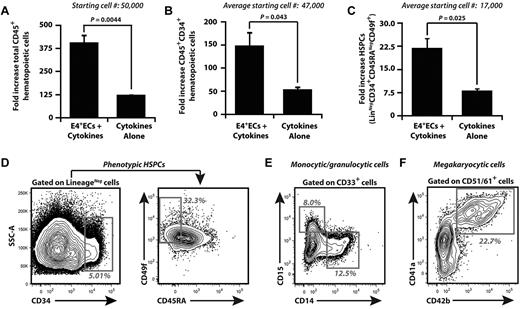
To determine whether hECs expand hCB, 5 × 104 CD34+ hCB was cultured in serum-free media with 50 ng/mL SCF, 50 ng/mL TPO, and 50 ng/mL Flt-3–ligand (STF) media without E4+ECs (cytokines alone) or with E4+ECs. CD34+ hCB cells were maintained in cumulative culture conditions (Figure 1A-F), where media was supplemented with STF cytokine cocktail every other day for 12 days. Nonadherent hematopoietic cells were plated on fresh E4+ECs with STF culture media when the cell density reached 2 × 106 cells/mL. Expanded hematopoietic cells were analyzed for total nucleated cells (TNCs; Figure 1A), CD45+CD34+ hematopoietic progenitor cells (HPCs; Figure 1B,D), and CD45+Lin−CD34hi+CD45RA−CD49f+ HSPCs (Figure 1C-D). We show that E4+ECs sustained cumulative expansion of TNCs by 400-fold, whereas cytokine-alone cultures expanded TNCs by approximately 125-fold (Figure 1A). E4+ECs expanded CD45+CD34+ HPCs (Figure 1B) by 150-fold of cocultured hCB with a 23-fold expansion of primitive Lin−CD34hi+CD45RA−CD49f+ HSPCs (Figure 1C). The cytokines alone supported expansion of HPCs by 50-fold with an approximately 7-fold expansion of HSPCs. E4+ECs fostered expansion of CD33+CD14+ and CD33+CD15+ myeloid (Figure 1E) and CD51/61+CD41a+CD42b+ megakaryocytic cells (Figure 1F). The increase in E4+EC–mediated expansion of CD34+ UCB was confirmed in 14 independent experiments, and ranges of expansion are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We assessed the ability of E4+EC feeder layers to expand CD34+ hCB under high-dose cytokines and found that E4+ECs enhanced the expansion of TNCs (∼ 1350-fold), HPCs (350-fold), and HSPCs (24-fold; supplemental Figure 1), approximately 3 times more than high cytokines-alone cultures. Therefore, these results demonstrate that ECs provide a cellular platform to expand and maintain hCB-derived HSPCs (hCB-HSPCs).
Serum- and cytokine-free EC cocultures supplemented with low doses of SCF, Flt3L, and TPO expand phenotypically marked HPCs/HSPCs and differentiated hematopoietic progenitors. Human umbilical cord blood (hCB) was enriched for CD34+ cells, and 5 × 104 CD34+ cells were cocultured with or without E4+ECs. (A-C) Cocultured hematopoietic cells were cumulatively expanded. Day 12 was chosen for analyses because of rapid attrition of hematopoietic cells, which were cultured without feeder cells. (A) Total hematopoietic cell expansion. (B) Total CD45+CD34+ HPC expansion. (C) Total phenotypically marked HSPC expansion defined by CD45+ Lin−CD34hi+CD45RA−CD49f+. Note that human hematopoietic cells that were cocultured with E4+ECs expanded significantly more compared with cultures without vascular feeder cells. (D) Representative contour plots of day 12 cumulative expansion of CD45+Lin−CD34hi+CD45RA−CD49f+ on E4+ECs. (E) Representative contour plots of day 12 cumulative expansion of CD33+CD14+ and CD33+CD15+ myeloid progenitors on E4+ECs. (F) Representative contour plots of day 12 cumulative expansion of CD51/61+CD41a+CD42b+ megakaryocytic progenitor cells on E4+ECs. Ranges of fold expansion are listed in supplemental Table 1. Results were analyzed using Student t test, and P < .05 was considered significant. Data are mean ± SD.
Serum- and cytokine-free EC cocultures supplemented with low doses of SCF, Flt3L, and TPO expand phenotypically marked HPCs/HSPCs and differentiated hematopoietic progenitors. Human umbilical cord blood (hCB) was enriched for CD34+ cells, and 5 × 104 CD34+ cells were cocultured with or without E4+ECs. (A-C) Cocultured hematopoietic cells were cumulatively expanded. Day 12 was chosen for analyses because of rapid attrition of hematopoietic cells, which were cultured without feeder cells. (A) Total hematopoietic cell expansion. (B) Total CD45+CD34+ HPC expansion. (C) Total phenotypically marked HSPC expansion defined by CD45+ Lin−CD34hi+CD45RA−CD49f+. Note that human hematopoietic cells that were cocultured with E4+ECs expanded significantly more compared with cultures without vascular feeder cells. (D) Representative contour plots of day 12 cumulative expansion of CD45+Lin−CD34hi+CD45RA−CD49f+ on E4+ECs. (E) Representative contour plots of day 12 cumulative expansion of CD33+CD14+ and CD33+CD15+ myeloid progenitors on E4+ECs. (F) Representative contour plots of day 12 cumulative expansion of CD51/61+CD41a+CD42b+ megakaryocytic progenitor cells on E4+ECs. Ranges of fold expansion are listed in supplemental Table 1. Results were analyzed using Student t test, and P < .05 was considered significant. Data are mean ± SD.
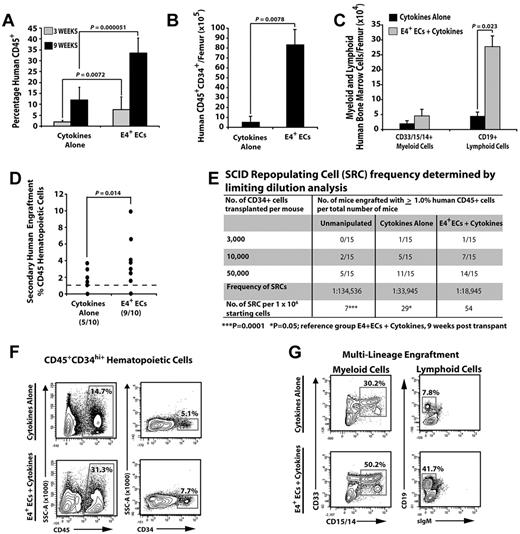
To assess whether E4+ECs were capable of expanding functional hCB-HSPCs with long-term hematopoietic engraftment potential, we separated the hematopoietic cells from E4+ECs and transplanted 1 × 106 unmanipulated, or day 12 cumulatively expanded cells from the cytokines-alone and E4+ECs cultures, separately into NSG mice (n = 4 independent experiments). Cocultured hCB-HSPCs on E4+ECs more efficiently engrafted human CD45+ cells at 3 and 9 weeks after transplantation than did hCB-HSPCs expanded with cytokines alone (3 weeks, 4-fold; and 9 weeks, 5-fold; Figure 2A,F). Furthermore, transplanted CD34+ hCB that was expanded on E4+ECs generated more CD45+CD34+ HSPCs in NSG mice compared with cytokines alone (16-fold greater; Figure 2B,F). Both cytokines alone and E4+EC cohorts were capable of multilineage engraftment, with E4+ECs having a far greater capacity to retain multilineage potential (Figure 2C,G). Transplantation with limiting dilution of E4+EC–derived CD34+ hCB showed a greater frequency of hCB cells capable of human CD45+ engraftment (> 1.0%) compared with cytokines alone (2-fold greater) and freshly isolated CD34+ hCB (7.5-fold greater; Figure 2E; n = 3 independent experiments).
Human CD34+ CBs cocultured with ECs give rise to multilineage engraftment in NSG mice. (A) Percentage of engrafted human CD45+ hematopoietic cells at 3 and 9 weeks after transplantation. (B) Total number of engrafted phenotypically marked human HPCs. (C) Total number of terminally differentiated mature cells per femur. (D) Percentage of human CD45 engraftment in secondary recipients transplanted with human CD45+ hematopoietic cells from primary recipients in panels A through C (N = 10 for each condition; 9 of 10 in the E4+EC group and 5 of 10 in the cytokines-alone group had significant human CD45 engraftment: > 1%). All human cells analyzed with the cell population that was gated on cells that were negative for mouse Ter119 and CD45. (E) A total of 50 000 CD45+CD34+ hCB cells were cultured with or without feeder layers in serum-free conditions and supplemented with 50 ng/mL SCF, 50 ng/mL TPO, and 50 ng/mL Flt3L for 12 days. CD45+CD34+ cells were isolated and transplanted into NSG mice with the corresponding cell doses. Unmanipulated CD45+CD34+ were used as controls. At 3 and 9 weeks after transplantation, mice were analyzed for human CD45+ cells in bone marrow. Mice with > 1% engraftment were considered positive. Mice that were positive at 3 weeks remained positive at 9 weeks, with higher levels of human CD45+ engraftment at 9 weeks. SCID-repopulating cells were determined using L-Calc Version 1.1 software. ***P = .0001. *P = .05. Reference group: E4+ECs + cytokines, analyzed 9 weeks after transplantation. (F) Representative contour plots of human HPC engraftment 9 weeks after transplantation. (G) Representative contour plots of multilineage human hematopoietic cell engraftment 9 weeks after transplantation. Note that human hematopoietic cells cocultured with E4+ECs have greater engraftment potential and give rise to a higher number of multilineage human hematopoietic progenitors and long-term engraftment in secondary recipients. Results were analyzed using the Student t test, and P < .05 was considered significant. Date are mean ± SD.
Human CD34+ CBs cocultured with ECs give rise to multilineage engraftment in NSG mice. (A) Percentage of engrafted human CD45+ hematopoietic cells at 3 and 9 weeks after transplantation. (B) Total number of engrafted phenotypically marked human HPCs. (C) Total number of terminally differentiated mature cells per femur. (D) Percentage of human CD45 engraftment in secondary recipients transplanted with human CD45+ hematopoietic cells from primary recipients in panels A through C (N = 10 for each condition; 9 of 10 in the E4+EC group and 5 of 10 in the cytokines-alone group had significant human CD45 engraftment: > 1%). All human cells analyzed with the cell population that was gated on cells that were negative for mouse Ter119 and CD45. (E) A total of 50 000 CD45+CD34+ hCB cells were cultured with or without feeder layers in serum-free conditions and supplemented with 50 ng/mL SCF, 50 ng/mL TPO, and 50 ng/mL Flt3L for 12 days. CD45+CD34+ cells were isolated and transplanted into NSG mice with the corresponding cell doses. Unmanipulated CD45+CD34+ were used as controls. At 3 and 9 weeks after transplantation, mice were analyzed for human CD45+ cells in bone marrow. Mice with > 1% engraftment were considered positive. Mice that were positive at 3 weeks remained positive at 9 weeks, with higher levels of human CD45+ engraftment at 9 weeks. SCID-repopulating cells were determined using L-Calc Version 1.1 software. ***P = .0001. *P = .05. Reference group: E4+ECs + cytokines, analyzed 9 weeks after transplantation. (F) Representative contour plots of human HPC engraftment 9 weeks after transplantation. (G) Representative contour plots of multilineage human hematopoietic cell engraftment 9 weeks after transplantation. Note that human hematopoietic cells cocultured with E4+ECs have greater engraftment potential and give rise to a higher number of multilineage human hematopoietic progenitors and long-term engraftment in secondary recipients. Results were analyzed using the Student t test, and P < .05 was considered significant. Date are mean ± SD.
To examine whether expanded CD34+ hCB from the 2 culture conditions could maintain their long-term engraftment and self-renewal potential, we transplanted human CD45+ cells from primary NSG mice 9 weeks after transplantation into secondary recipient NSG mice (n = 10 mice/culture condition) and then analyzed the secondary recipients 9 weeks later for human CD45+ engraftment (> 1%). We found that 9 of 10 mice from EC coculture conditions and 5 of 10 mice from the cytokines-alone condition sustained long-term engraftment of human hematopoietic cells (> 1%), with the EC-expanded cells resulting in a greater average percentage of human CD45+ cells (Figure 2D) in the engrafted mice. Therefore, ECs support the expansion of CD34+ hCB that are capable of rapid and sustainable human multilineage hematopoietic engraftment of NSG mice.
hCB are clinically useful hematopoietic cells for therapeutic HSPC transplantation. However, the total number of functional and engraftable HSPCs within the hCB still remains the limiting variable that strongly impacts transplantation success. Identifying methods for robust expansion of hCB-derived HSPCs would greatly expand the pool of potential donors and is expected to reduce the morbidity and mortality of hCB transplantation. However, defining a set of growth factors and cytokines that can expand long-term engraftable human HSCs has proven to be difficult. Here we show that hECs provide proper cues that enable expansion of CD34+ hCB-derived HSPCs, which are capable of rapidly reconstituting the hematopoietic system of NSG mice and can maintain long-term engraftment in secondary transplantation assays compared with EC-free cultures. Indeed, ECs provide many of the necessary angiocrine growth factors that balance the expansion of engraftable hCB cells and lineage-specific differentiation of HSPCs in vivo.8,10-14 Because ECs also support the lineage specific differentiation of hCB progenitors, this platform could be tuned for enhanced engraftment of granulocytic or megakaryopoietic progenitors to speed blood count recovery after transplantation. Therefore, ECs may prove to be an ideal cellular platform to expand hCB and allow identification of new growth factors that could collectively regulate expansion, maintenance, and differentiation of human HSPCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
J.M.B. and S.R. were supported by Ansary Stem Cell Institute, Howard Hughes Medical Institute, Empire State Stem Cell Board, and New York State Department of Health (grants NYSTEM, C024180, C026438, and C026878), the National Heart, Lung, and Blood Institute (R01 HL097797), Qatar National Priorities Research Foundation (NPRP08-663-3-140), and Qatar Foundation BioMedical Research Program (BMRP).
National Institutes of Health
Authorship
Contribution: J.M.B. and E.J.G. purified, expanded, and transplanted cells; D.J.J. and D.J.N. performed and analyzed FACS; J.M.B., J.M.S., and S.R. designed, coordinated, and supervised experimental procedures; and J.M.B. and S.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jason M. Butler, Department of Genetic Medicine, Weill Cornell Medical College, 1300 York Ave, A869A, New York, NY, 10065; e-mail: jmb2009@med.cornell.edu; and Shahin Rafii, Department of Genetic Medicine, Weill Cornell Medical College, 1300 York Ave, A863A, New York, NY, 10065; e-mail: srafii@med.cornell.edu.
References
Author notes
J.M.B. and E.J.G. contributed equally to this study.