Abstract
Ly6G is a glycosylphosphatidylinositol (GPI)–anchored protein of unknown function that is commonly targeted to induce experimental neutrophil depletion in mice. In the present study, we found that doses of anti-Ly6G Abs too low to produce sustained neutropenia remained capable of inhibiting experimental arthritis, leaving joint tissues free of infiltrating neutrophils. Thioglycollate-stimulated peritonitis was also attenuated. No alteration in neutrophil apoptosis was observed, implicating impaired recruitment. Indeed, Ly6G ligation abrogated neutrophil migration toward LTB4 and other chemoattractants in a transwell system. Exploring the basis for this blockade, we identified colocalization of Ly6G and β2-integrins by confocal microscopy and confirmed close association by both coimmunoprecipitation and fluorescence lifetime imaging microscopy. Anti-Ly6G Ab impaired surface expression of β2-integrins in LTB4-stimulated neutrophils and mimicked CD11a blockade in inhibiting both ICAM-1 binding and firm adhesion to activated endothelium under flow conditions. Correspondingly, migration of β2-integrin–deficient neutrophils was no longer inhibited by anti-Ly6G. These results demonstrate that experimental targeting of Ly6G has functional effects on the neutrophil population and identify a previously unappreciated role for Ly6G as a modulator of neutrophil migration to sites of inflammation via a β2-integrin–dependent mechanism.
Introduction
Neutrophils are one of the first cell types to reach sites of infection or acute inflammation. Recruitment involves an orchestrated sequence of events in which circulating neutrophils respond to chemotactic signals to become firmly adherent to activated endothelium, followed by transendothelial migration via either a paracellular or transcellular route.1,2 Once at a site of inflammation, neutrophils contribute to ongoing leukocyte recruitment and tissue injury by releasing lipid mediators, proteases, reactive oxygen species (ROS), and other factors.3-5 Whereas they are critical to immune defense, neutrophils can, also play a pathogenic role in chronic inflammatory diseases and therefore their recruitment is subject to numerous levels of control, not all of which are understood completely.1 Delineating these regulatory pathways will provide insights into the mechanisms of tissue injury in inflammatory disorders and novel targets for drug development.
Much of the experimental evidence implicating neutrophils in disease has been obtained through murine studies in which this lineage was selectively depleted using Abs that bind the neutrophil surface antigen Ly6G, such as RB6-8C5 (more typically termed anti–Gr-1).6,7 However, the function of Ly6G remains unknown. Ly6G is a small protein of approximately 25 kDa that is tethered to the cell surface via a GPI linker.7 In bone marrow (BM), peripheral blood, and wound exudates, the expression of Ly6G is limited to cells with granulocyte morphology.7,8 Structurally, Ly6G belongs to the Ly6/urokinase plasminogen activator receptor (uPAR) family of proteins featuring a “3 finger fold” motif stabilized by 4-5 disulfide bonds.9 These protein folds are believed to create a docking site for other molecules. However, no ligand for Ly6G has been identified. Furthermore, whereas other members of the Ly6/uPAR family have been implicated in signaling, presumably by association with transmembrane proteins, no signaling function for Ly6G has been defined.9-11 Ly6G is expressed only in mice, but a structurally related Ly6/uPAR family member, CD177 (also called HNA-2a, NB1, or PRV-1), is expressed in human neutrophils and has been implicated in some cases of human immune neutropenia.12 CD177 has a direct association with β2-integrins,12-14 is a counterreceptor for PECAM-1, and Abs against CD177 impede neutrophil migration across an endothelial barrier.15 The extent of the functional homology between murine and human proteins of the Ly6/uPAR family remains undefined.
Recently, data have begun to emerge showing that ligation of Ly6G via specific Abs may have functional consequences beyond physical depletion. TNFα-primed mice given anti–Gr-1 (which binds both Ly6G and the structurally related protein Ly6C)7,8 or the Ly6G-specific Ab 1A8 (hereafter referred to as anti-Ly6G) develop disseminated intravascular coagulation and death.16,17 In vitro, cross-linking using anti–Gr-1 F(ab′)2 fragments and a secondary Ab induces up-regulation of neutrophil CD11b and a modest rise in F-actin, but whether this phenomenon reflects binding of Ly6G, Ly6C, or both is unknown.17 Anti–Gr-1 administration has also been associated with the appearance of apoptotic-appearing neutrophils in inflammatory infiltrates, giving rise to the hypothesis that anti–Gr-1 induces apoptosis in circulating or inflammatory neutrophils but not in their BM precursors, potentially because of differential expression of the antiapoptotic Bcl-2 family protein Mcl-1.18
In human arthritis, neutrophils are invariably present in the inflamed joint.19,20 The pathogenic contribution of these cells has been confirmed experimentally in conventional depletion experiments using anti–Gr-1, as well as by studies in genetically neutropenic mice.20-23 While investigating the effect of partial neutropenia in murine K/BxN arthritis, we made the unexpected observation that low doses of anti–Gr-1 with minimal sustained effect on circulating neutrophil counts nevertheless led to dramatic attenuation of disease. Subsequent studies using anti-Ly6G confirmed this observation and identified impairment of neutrophil migration rather than accelerated apoptosis as the most plausible explanation. We found that Ly6G associated closely with β2-integrins and that anti-Ly6G attenuated both β2-integrin expression and function, a role implicated in the efficacy of Ab treatment. These results imply a previously unappreciated function for this highly expressed neutrophil protein, suggesting a novel mechanism regulating neutrophil recruitment to inflamed sites. Further, they demonstrate previously unrecognized similarities with CD177 that render the study of Ly6G potentially informative as to this nonhomologous human counterpart.
Methods
Reagents
Endotoxin-free anti–Gr-1, anti-Ly6G, and isotype control (rat IgG2a, clone 2A3) were from BioXCell. Endotoxin-free anti-Ly6B.2 (clone 7/4) was from AbD Serotec. Anti-CD18, anti-CD11b–FITC, H2DCFDA, phalloidin–Alexa Fluor 488, and live/dead staining reagent were from Invitrogen. CD45-PE-Cy7 and annexin V staining reagents were from BD Pharmingen. Anti-CD11a–Alexa Fluor 488, anti–CD11a-biotin, anti-Ly6G–Alexa Fluor 647, anti–CD11a-PE, anti-CD11b–Alexa Fluor 488, anti–Ly6G-biotin, anti-Ly6G–Alexa Fluor 647, anti-CD62L–allophycocyanin, and anti–Gr-1–Alexa Fluor 647 were from BioLegend. Streptavidin-Cy3 and streptavidin–Alexa Fluor 594 were from Jackson ImmunoResearch Laboratories. NIMP-R14 was from Abcam. Phorbol 12-myristate 13-acetate (PMA), murine TNFα, and staurosporine were from Sigma-Aldrich. LTB4 was from Cayman Chemical.
Mice
Wild-type C57BL/6J (B6), B6;129S4-Fcgr2btm1Ttk/J (FcγRII−/−), and B6.129S7-Itgb2tm2Bay/J (CD18−/−) mice were obtained from The Jackson Laboratory. K/BxN mice expressing the TCR transgene KRN and the MHC class II molecule Ag7 were generated at a dedicated colony maintained at The Jackson Laboratory. Experiments were approved by the animal care and use committee of the Dana-Farber Cancer Institute.
Experimental arthritis
Arthritogenic serum was obtained from 9- to 11-week-old K/BxN donors by cardiac puncture as described previously.24 Arthritis was initiated with IP injections of 150 μL of K/BxN serum on experimental days 0 and 2. To measure severity of disease, each paw was graded on a scale of 0-3 for a total score of 0-12 per mouse. Normal paws were scored 0; 1 additional point was given for swelling affecting the dorsal surface, ventral surface, ankle/wrist, and toes, capping at a maximum of 3.25 “Flare” (transient paw swelling) was assessed on the same scale 30 minutes after serum administration.26 Histologic assessment of articular neutrophils was accomplished using NIMP-R14 Ab staining.27 Interpretability of staining in the context of anti-Ly6G administration was confirmed by intact staining of BM cells with neutrophil morphology in the sections analyzed.
Experimental peritonitis
Peritonitis was initiated by instillation of 1 mL of sterile aged 3% thioglycollate (TG) broth intraperitoneally 4 hours before harvest. The peritoneum was lavaged with 2 mL of PBS and cells were counted by flow cytometry using synthetic 15-μm microspheres (Polysciences) to normalize counting between specimens (Polysciences). For apoptosis studies, peritoneal cells were harvested with warm RPMI/0.1% FBS.
Neutrophil quantitation
For neutrophil enumeration, blood was harvested by cardiac puncture from lethally anesthetized mice and transferred immediately into EDTA-containing Vacutainer tubes (BD Medical). A complete blood count and automated leukocyte differential was obtained using an Advia 120 Hematology System (Siemens) and appropriate species-specific standards and software. The accuracy of the automated neutrophil assessment was confirmed previously by parallel flow cytometric examination.28 BM cells were obtained by femoral flush and CD45+/Gr-1+/CD11bhi cells quantitated as a proportion of total CD45+ cells. Reliability of anti–Gr-1 staining in the context of anti-Ly6G administration was determined by in vitro experiments in which preincubation with anti-Ly6G (1-20 μg/mL) decreased anti–Gr-1–Alexa Fluor 647 mean fluorescence intensity (MFI) by up to 40% but did not impair neutrophil identification because of their very high baseline mean fluorescence intensity (data not shown). Methods for defining maturity of circulating neutrophils are included in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Studies of neutrophil migration and activation
All media and centrifuges were maintained at room temperature (RT). For migration studies, BM was harvested by flushing with RPMI medium, passing through a 100-μm filter, centrifuging for 3 minutes at 300g, and resuspending in RPMI medium containing 0.1% heat-inactivated FBS. Cell suspensions were exposed to anti-Ly6G or isotype for 10 minutes at RT. Thereafter, 500 μL of cells was placed in the upper compartment of a transwell chamber featuring 3-μm pores in an uncoated polycarbonate membrane (Corning), and 1.5 mL of 0.1% FBS RPMI medium containing LTB4 10−7M or vehicle was added to the bottom chamber. After incubation for 3 hours at 37°C and 5% CO2, the lower chamber was harvested; CD45+/Gr-1+/CD11bhi cells were enumerated using flow cytometry by comparison with counting beads added to each sample. Fab fragments for transwell studies were generated using a commercial kit (Pierce). To measure intracellular ROS, BM cells (4 × 106/mL) were incubated with 10μM H2DCFDA in HBSS with Ca2+/Mg2+ for 15 minutes at 37°C. Cells were then washed and seeded at 4 × 105 cells per well in a 96-well microplate in the presence or absence of H2O2 (0.25μM), PMA (1μM), and isotype Ab or anti-Ly6G (10 μg/mL). Dichlorodihydrofluorescein fluorescence was monitored in 25-second intervals for 60 minutes using a fluorescent plate reader with an excitation wavelength of 480 nm and an emission wavelength of 530 nm. Actin polymerization was assessed in permeabilized cells by staining for F-actin with phalloidin–Alexa Fluor 488.29 Briefly, after incubation with PMA (1μM), 2A3 (10 μg/mL), or anti-Ly6G (10 μg/mL) for 10 minutes, BM cells were washed with ice-cold FACS buffer and stained for neutrophil surface markers. Cells were then fixed with 4% paraformaldehyde in PBS for 15 minutes, permeabilized with 0.5% Triton X-100 for 10 minutes, and stained with labeled phalloidin in FACS buffer containing 0.05% saponin.
Isolation of mouse neutrophils from BM
Mouse neutrophils were isolated by flushing femurs and tibias with RPMI medium containing 0.1% FBS. After depletion of erythrocytes using ammonium chloride potassium lysis buffer, cells were resuspended in Ca/Mg-free HBSS buffer, loaded on a discontinuous Percoll density gradient (63%/81%), and centrifuged at 1000g for 30 minutes. This procedure routinely yielded > 90% neutrophils as determined by flow cytometry.
ICAM-1-Fc–binding assay
The ICAM-1-Fc–binding assay was described previously.30 Briefly, ICAM-1-Fc (R&D Systems) or Fc control (Bethyl laboratories) was incubated with goat anti–human Fc-specific IgG F(ab′)2-FITC at 4°C for 30 minutes; 25 μg/mL of the ICAM-1-Fc-F(ab′)2-FITC was applied to neutrophils at 4°C for 30 minutes to detect active β2-integrins.
Flow chamber assay
Murine endothelial bEND5 cells were grown to confluence in 60-mm culture dishes and activated for at least 3 hours with 5mM murine TNFα as described previously.31 Neutrophils purified from B6 BM by density centrifugation were treated with isotype Ab or anti-Ly6G (10 μg/mL at RT for 10 minutes), resuspended at 2 × 106 cells/mL in HBSS and 10mM HEPES with either 2mM Ca2+/2mM Mg2+ or 5mM EDTA, and introduced into the flow assay chamber. Tethering of neutrophils was observed at low physiologic wall shear stress (0.5 dyne/cm2 for 1 minute). Firm adhesion was observed at high physiologic shear stress (3 minutes pause, 0.5 dyne/cm2 for 1 minute, followed by stepwise increases to 10 dyne/cm2) as described previously.32
Immunofluorescence and confocal microscopy
Cytospin preparations of BM cells (2 × 105/sample) were fixed with 4% paraformaldehyde in PBS for 10 minutes and permeabilized with −20°C methanol for 3 minutes. After blocking with 10% FBS in PBS for 1 hour at RT, cells were stained for CD11a, CD11b, and Ly6G with the primary Abs anti–CD11a-biotin, anti-CD11b–Alexa Fluor 488, and anti-Ly6G–Alexa Fluor 647 for 1 hour at RT, followed by the secondary streptavidin-Cy3 for 30 minutes at RT. Immunofluorescence images were taken by a confocal microscope (C1; Nikon) using a Plan Apochromat 60×/1.40 numerical aperture oil objective (Nikon) in high-resolution format at 1024 × 1024 pixels. The images were acquired and analyzed using Nikon EZ-C1 software.
FLIM
Protein interactions were determined by fluorescence lifetime imaging microscopy (FLIM) using a time-correlated single-photon counting confocal FLIM system Nikon TiE microscope with a Plan Apochromat VC 60×/1.4 DC N2 objective).33,34 Cells were cytospun, fixed, permeabilized, and stained for fluorescence resonance energy transfer (FRET) donor-acceptor pairs as indicated. Alexa Fluor 488 (detecting CD11a or CD11b) served as the donor fluorophore and Alexa Fluor 594 (detecting Ly6G or CD45) served as the acceptor fluorophore. Data were imported to Adobe Illustrator, and the contrast and brightness were adjusted equally for all images. Specific protocols used for FLIM are provided in supplemental Methods.
In situ cross-linking and coimmunoprecipitation
Cross-linking of protein complex was performed in cells using 3mM dimethyl 3,3′-dithiobispropionimidate in conjugation buffer (20mM sodium phosphate, 150mM NaCl, pH 8.0) for 45 minutes at RT, followed by adding Tris-HCl, pH 8.0, at the final concentration of 50mM to quench the reaction. Cells were extensive washed with PBS, and then lysed in NP40 lysis buffer (Boston BioProducts) to extract protein. For immunoprecipitation, the specified Ab and Protein G Sepharose (GE Healthcare) were added to clarified lysate and incubated for 2 hours at 4°C with rotation. Beads were washed with lysis buffer, resuspended in 2× Laemmli sample buffer (Boston BioProducts) with 100mM dithiothreitol, and incubated at 37°C for 15 minutes before boiling to cleave disulfide bonds within the cross-linker. Immunoprecipitants were separated by SDS-PAGE and analyzed by immunoblotting.
Statistical analysis
Error bars depict SD, with the exception of the arthritis clinical score plots and FLIM analysis, where they reflect SEM. P values were calculated using the Student t test unless otherwise indicated in figure legends. P < .05 was considered significant.
Results
Anti–Gr-1 blocks K/BxN serum transfer arthritis at nondepleting doses
In K/BxN serum transfer arthritis, parenteral administration of serum containing IgG autoantibodies directed against the glycolytic enzyme glucose-6-phosphate isomerase results in rapid onset of destructive joint inflammation (synovitis) in most recipient strains of mice. The essential role of neutrophils in this process was demonstrated previously by studies in which repeated administration of anti–Gr-1 (250 μg intraperitoneally) was found to both prevent and treat disease.20 In the present study, to determine the relative contribution of various innate immune lineages to synovitis, we sought to generate incomplete neutropenia using lower doses of Ab. Anti–Gr-1 was administered intraperitoneally to B6 mice on day −1 and day 2 relative to K/BxN serum 150 μL on days 0 and 2. Surprisingly, we found that anti–Gr-1 doses as low as 5 μg could completely abolish arthritis (Figure 1A). Moreover, suppression of arthritis was observed despite the persistence of serum-induced acute vascular edema (“flare”), a phenotype that requires the presence of a functional pool of circulating neutrophils35-37 (Figure 1B). Measurement of neutrophil numbers in blood confirmed this observation and showed that, on arthritis day 4, animals treated with low-dose anti–Gr-1 demonstrated numbers of circulating neutrophils comparable with those from healthy mice (Figure 1C). These results suggested that suppression of arthritis by anti–Gr-1 was not entirely dependent on depletion of circulating neutrophils, but might also be mediated by altered neutrophil function.
Anti–Gr-1 blocks K/BxN serum transfer arthritis at nondepleting doses. (A) Mice were administered anti–Gr-1 intraperitoneally at various doses, followed by arthritogenic K/BxN serum as indicated. Data derived from 2-5 experiments (n = 7-21 mice/group, all P < .01 vs control by ANOVA). (B) Paw edema scored 30 minutes after injection of K/BxN serum. Neutrophil-dependent edema was suppressed only at high doses of anti–Gr-1. Data are presented from 7-21 mice per condition as described in panel A. Control indicates PBS in 4 experiments. (C) Automated absolute neutrophil counts in blood from unmanipulated mice and mice killed on arthritis day 4. Animals treated twice with 10 μg of anti–Gr-1 exhibited lower neutrophil counts compared with control animals with inflamed joints, but similar numbers to noninflamed mice. *P < .05; ***P < .001.
Anti–Gr-1 blocks K/BxN serum transfer arthritis at nondepleting doses. (A) Mice were administered anti–Gr-1 intraperitoneally at various doses, followed by arthritogenic K/BxN serum as indicated. Data derived from 2-5 experiments (n = 7-21 mice/group, all P < .01 vs control by ANOVA). (B) Paw edema scored 30 minutes after injection of K/BxN serum. Neutrophil-dependent edema was suppressed only at high doses of anti–Gr-1. Data are presented from 7-21 mice per condition as described in panel A. Control indicates PBS in 4 experiments. (C) Automated absolute neutrophil counts in blood from unmanipulated mice and mice killed on arthritis day 4. Animals treated twice with 10 μg of anti–Gr-1 exhibited lower neutrophil counts compared with control animals with inflamed joints, but similar numbers to noninflamed mice. *P < .05; ***P < .001.
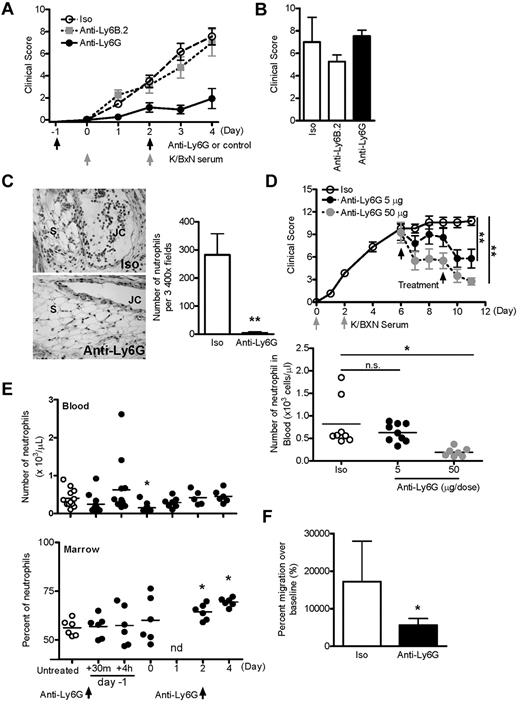
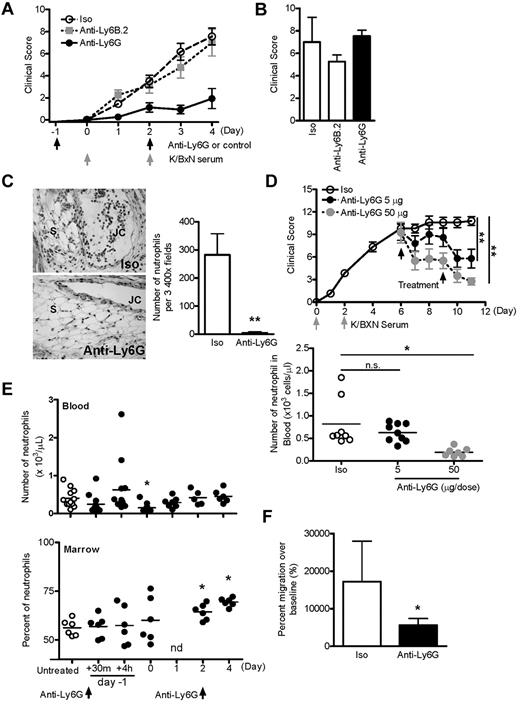
Ly6G-selective Ab attenuates arthritis and inhibits neutrophil recruitment in vivo
Although used widely in neutrophil studies, anti–Gr-1 also recognizes Ly6C, an antigen expressed on monocytes and other lineages. Correspondingly, high-dose anti–Gr-1 depletes monocytes in addition to neutrophils.8 For further investigation, we therefore used the Ly6G-selective Ab 1A8.7,8 Consistent with our earlier results, we found that 5 μg of anti-Ly6G induced blockade of K/BxN arthritis without abrogation of the neutrophil-dependent flare (Figure 2A-B). In contrast, neither nonbinding isotype control (2A3) nor isotype-matched neutrophil-binding Ab anti-Ly6B.2 exhibited a similar effect. Histologic assessment at day 4 demonstrated the absence of neutrophils from ankle tissue (Figure 2C). Further, administration of anti-Ly6G could attenuate established arthritis (Figure 2D). To exclude transient but profound neutropenia as a basis for arthritis suppression, we assessed circulating and BM neutrophils in otherwise unmanipulated mice treated with anti-Ly6G. At the 5-μg experimental dose, circulating neutrophils exhibited a transient reduction of approximately 60% 24 hours after Ab injection, but otherwise remained intact (Figure 2E). Even at the 24-hour time point, more than half of experimental mice demonstrated circulating neutrophil concentrations within the range of the normal controls. BM neutrophils were not depleted, and indeed increased with time, as reported previously by others.18 Examination of peripheral blood neutrophils after anti-Ly6G treatment showed that > 93% of the circulating neutrophils were morphologically mature (supplemental Figure 1). These results suggested that neither the reduced availability of neutrophils nor the replacement of mature neutrophils by immature neutrophils was sufficient for suppression of arthritis. To further confirm this observation, we used a model of neutrophilic inflammation that progresses more rapidly than arthritis: TG peritonitis.38 At the equivalent of day 4, when circulating neutrophil counts were unequivocally normal, mice treated twice with 5 μg of anti-Ly6G exhibited clear impairment of neutrophil recruitment to the TG-stimulated peritoneum (Figure 2F).
Anti-Ly6G attenuates arthritis and inhibits neutrophil recruitment in joints and peritoneum. (A) Arthritic response of mice given 5 μg of Ab twice and 150 μL of K/BxN serum (rat IgG2a isotype, n = 14; anti-Ly6B.2, n = 8; anti-Ly6G, n = 12 pooled from 2-6 experiments). Shown is isotype (Iso) versus anti-Ly6G. P < .0001 by ANOVA. (B) K/BxN serum–induced flare was assessed 30 minutes after the first injection of K/BxN serum using the same scoring system as in panel A. (C) Representative sections of day-4 ankle histopathology from mice receiving isotype control Ab or anti-Ly6G (H&E stain; 20× objective). JC indicates joint cavity; and S, synovium. Right panel shows the quantification of neutrophils per 3 high-power field of day-4 ankle tissue (n = 12-18 ankles). (D) Treatment of active arthritis with nondepleting and depleting doses of anti-Ly6G. Two doses of anti-Ly6G at 5 or 50 μg/dose or control Ab at 50 μg/dose were administered intraperitoneally on days 6 and 9 of active arthritis. Circulating neutrophils were enumerated by automated cytometer on day 11. (E) Effect of anti-Ly6G (5 μg intraperitoneally on days −1 and 2) on neutrophils in the blood and BM of otherwise unmanipulated mice (n = 5-12 mice/time point from 4 experiments). There was a trend toward decreased neutrophil counts in blood 30 minutes after injection (P = .12). nd indicates not done. (F) Anti-Ly6G (5 μg) was administered intraperitoneally to nonarthritic mice and TG instilled intraperitoneally at the equivalent of day 4 of arthritis. Peritoneal cells were harvested at 4 hours by lavage and neutrophils enumerated by flow cytometry. Data are from n = 6 animals per group from 2 experiments. *P < .05; **P < .01.
Anti-Ly6G attenuates arthritis and inhibits neutrophil recruitment in joints and peritoneum. (A) Arthritic response of mice given 5 μg of Ab twice and 150 μL of K/BxN serum (rat IgG2a isotype, n = 14; anti-Ly6B.2, n = 8; anti-Ly6G, n = 12 pooled from 2-6 experiments). Shown is isotype (Iso) versus anti-Ly6G. P < .0001 by ANOVA. (B) K/BxN serum–induced flare was assessed 30 minutes after the first injection of K/BxN serum using the same scoring system as in panel A. (C) Representative sections of day-4 ankle histopathology from mice receiving isotype control Ab or anti-Ly6G (H&E stain; 20× objective). JC indicates joint cavity; and S, synovium. Right panel shows the quantification of neutrophils per 3 high-power field of day-4 ankle tissue (n = 12-18 ankles). (D) Treatment of active arthritis with nondepleting and depleting doses of anti-Ly6G. Two doses of anti-Ly6G at 5 or 50 μg/dose or control Ab at 50 μg/dose were administered intraperitoneally on days 6 and 9 of active arthritis. Circulating neutrophils were enumerated by automated cytometer on day 11. (E) Effect of anti-Ly6G (5 μg intraperitoneally on days −1 and 2) on neutrophils in the blood and BM of otherwise unmanipulated mice (n = 5-12 mice/time point from 4 experiments). There was a trend toward decreased neutrophil counts in blood 30 minutes after injection (P = .12). nd indicates not done. (F) Anti-Ly6G (5 μg) was administered intraperitoneally to nonarthritic mice and TG instilled intraperitoneally at the equivalent of day 4 of arthritis. Peritoneal cells were harvested at 4 hours by lavage and neutrophils enumerated by flow cytometry. Data are from n = 6 animals per group from 2 experiments. *P < .05; **P < .01.
Search for a functional impact of Ly6G ligation
Neutrophil involvement in murine autoantibody-mediated arthritis proceeds in 4 stages. First, neutrophils cooperate with mast cells to mediate joint-selective vascular permeability (flare) that facilitates entry of autoantibodies.35-37 Second, neutrophils migrate into the joint, recruited by chemoattractants including LTB4, C5a, and others.4,23,39 Third, having entered the joint tissues, neutrophils promote inflammation and tissue injury via elaboration of a wide range of cytokines, chemokines, lipid mediators, and proteases.3,4,19,23,40 Finally, neutrophils die by apoptosis and their debris is removed by local macrophages.38,41 In our arthritis model, anti-Ly6G left neutrophil-mediated vascular leak intact, but neutrophils were no longer evident in joint tissues (Figure 2A-C). We therefore focused our investigation on neutrophil death and migration.
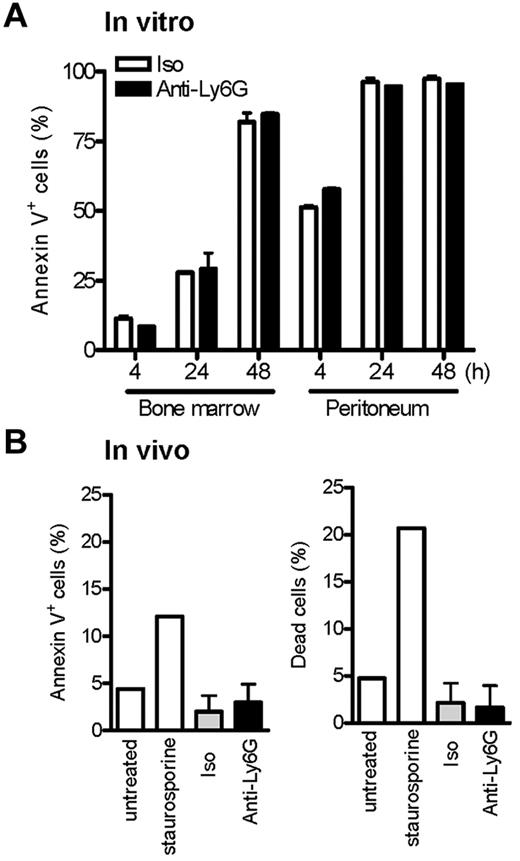
Ab ligation of Ly6G at low doses does not induce neutrophil apoptosis
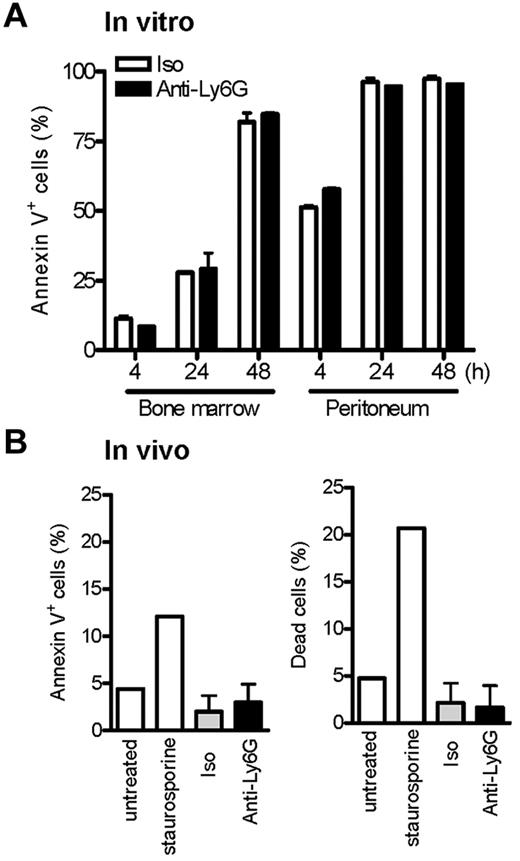
Recently, Ribechini et al reported that instillation of anti–Gr-1 (250 μg intraperitoneally) after recruitment of neutrophils to peritoneum via IP TG resulted in the depletion of neutrophils and the appearance of pyknotic nuclei, suggesting induction of apoptosis.18 Indeed, accelerated neutrophil apoptosis is a recognized basis for resistance to experimental arthritis.38,41 We therefore considered whether low-dose anti-Ly6G might induce apoptosis of neutrophils activated in the process of recruitment. To explore this possibility, we exposed BM neutrophils and neutrophils obtained from TG-stimulated peritoneum to anti-Ly6G or control. Apoptosis was assessed over the course of 48 hours by staining for annexin V. Whereas, as expected, activated neutrophils entered apoptosis more rapidly, no specific proapoptotic effect of anti-Ly6G was observed (Figure 3A). To confirm this observation in vivo, we pretreated animals with anti-Ly6G and induced TG peritonitis to look for excess apoptosis in recruited neutrophils, as is readily apparent when TG peritonitis is induced in Foxo3a−/− mice susceptible to accelerated apoptosis38 ; again, no difference in apoptosis could be observed (Figure 3B). These results are consistent with the lack of pyknotic neutrophils in anti-Ly6G–treated day-4 ankle tissues (Figure 2C). We therefore suspect that the proapoptotic mechanism defined by Ribechini et al using high-dose anti–Gr-1 Ab is not decisive for suppression of arthritis by low-dose anti-Ly6G.
Ab ligation of Ly6G at low doses does not induce neutrophil cell death in vitro and in vivo. (A) Cells from whole BM and TG-stimulated peritoneum were cultured with isotype or anti-Ly6G at 10 μg/mL, and neutrophil apoptosis was assessed at the indicated time points by annexin V. (B) Anti-Ly6G (5 μg) was administered intraperitoneally to mice as per the arthritis protocol and TG was instilled intraperitoneally at the equivalent of day 4 of arthritis. Peritoneal cells were harvested at 4 hours by lavage and neutrophils stained for annexin V and the percentage of live/dead cells. Negative and positive controls for cell apoptosis/death were BM cells cultured in the absence or presence of the apoptosis inducer staurosporine (3μM) for 4 hours. Results are representative of 2 independent experiments.
Ab ligation of Ly6G at low doses does not induce neutrophil cell death in vitro and in vivo. (A) Cells from whole BM and TG-stimulated peritoneum were cultured with isotype or anti-Ly6G at 10 μg/mL, and neutrophil apoptosis was assessed at the indicated time points by annexin V. (B) Anti-Ly6G (5 μg) was administered intraperitoneally to mice as per the arthritis protocol and TG was instilled intraperitoneally at the equivalent of day 4 of arthritis. Peritoneal cells were harvested at 4 hours by lavage and neutrophils stained for annexin V and the percentage of live/dead cells. Negative and positive controls for cell apoptosis/death were BM cells cultured in the absence or presence of the apoptosis inducer staurosporine (3μM) for 4 hours. Results are representative of 2 independent experiments.
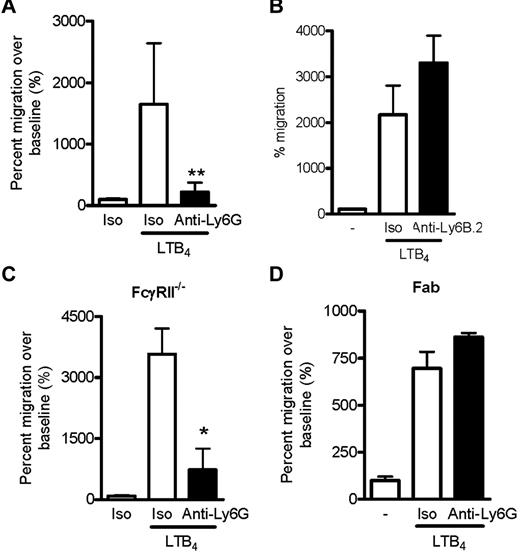
Anti-Ly6G inhibits LTB4-induced neutrophil migration
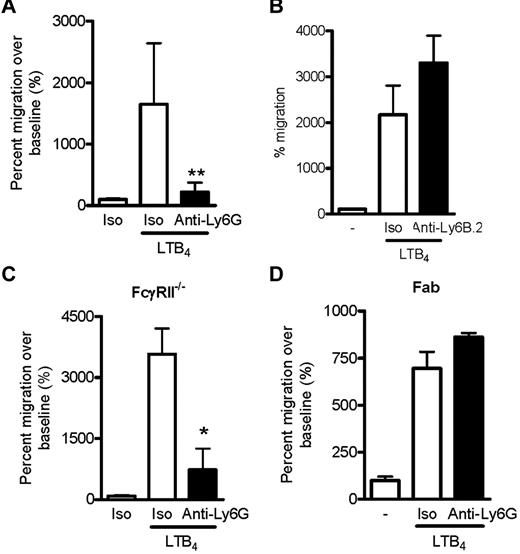
We next evaluated whether anti-Ly6G might impair neutrophil migration. Unfractionated, washed BM cells were preincubated with anti-Ly6G or isotype control and placed into the upper chamber of a transwell apparatus. Medium containing LTB4, a chemoattractant required for initial migration of neutrophils to the joint,39 was introduced into the lower chamber and migrating neutrophils were enumerated by flow cytometry of cells harvested from the lower chamber after 3 hours. Strikingly, anti-Ly6G specifically abrogated neutrophil migration in this system (Figure 4A). In contrast, the isotype-matched Ab anti-Ly6B.2 did not inhibit migration (Figure 4B), excluding the possibility that the effect of anti-Ly6G arose simply because of neutrophil binding. Impaired migration was also found for C5a, f-Met-Leu-Phe, and GM-CSF (supplemental Figure 2). To ensure that inhibition was not related to engagement of the inhibitory Fc receptor FcγRII by tethered Ab, we replicated the experiment in FcγRII−/− cells and found an identical result (Figure 4C). The anti-Ly6G effect was also preserved in density-purified neutrophils that were free of associated or attached platelets as assessed by CD41 staining (supplemental Figure 3). Interestingly, whereas Fab fragments from anti-Ly6G still bound well to neutrophils, they failed to inhibit migration, implicating oligomerization of Ly6G in the mechanism of inhibited migration (Figure 4D and supplemental Figure 4).
Ly6G Ab 1A8 inhibits LTB4-induced neutrophil migration. (A) LTB4 induced-migration of CD45+Gr-1+CD11bhi neutrophils in B6 BM after isotype or anti-Ly6G preincubation (10 μg/mL for 10 minutes) expressed as the percentage of migration in the absence of chemoattractant. Data presented are from 4 independent experiments. (B) The isotype-matched binding control anti-Ly6B.2 (10 μg for 10 minutes) did not affect neutrophil migration toward LTB4. (C) Migration assay using BM deficient in FcγRII, demonstrating preserved inhibition of migration. (D) Effect of anti-Ly6G Fab fragments (10 μg/mL for 10 minutes) on neutrophil migration. In panels B-D, data are representative of 2 independent experiments with similar results. *P < .05; **P < .01.
Ly6G Ab 1A8 inhibits LTB4-induced neutrophil migration. (A) LTB4 induced-migration of CD45+Gr-1+CD11bhi neutrophils in B6 BM after isotype or anti-Ly6G preincubation (10 μg/mL for 10 minutes) expressed as the percentage of migration in the absence of chemoattractant. Data presented are from 4 independent experiments. (B) The isotype-matched binding control anti-Ly6B.2 (10 μg for 10 minutes) did not affect neutrophil migration toward LTB4. (C) Migration assay using BM deficient in FcγRII, demonstrating preserved inhibition of migration. (D) Effect of anti-Ly6G Fab fragments (10 μg/mL for 10 minutes) on neutrophil migration. In panels B-D, data are representative of 2 independent experiments with similar results. *P < .05; **P < .01.
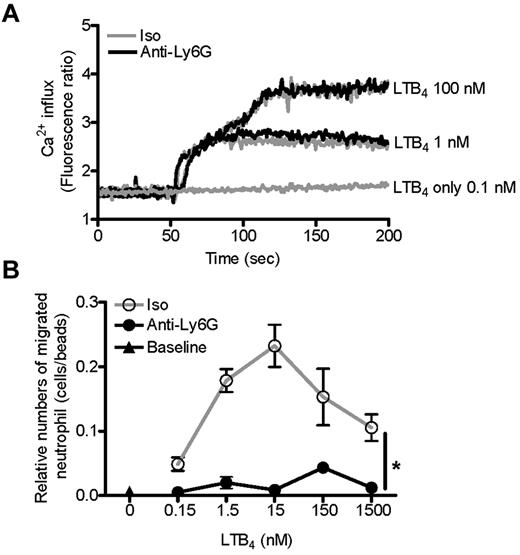
Migratory blockade is not due to a change in LTB4 receptor sensitivity or initial signal transduction
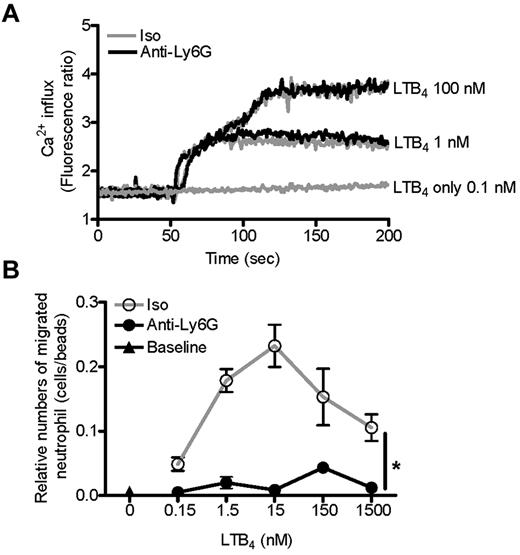
Neutrophil migration begins with detection of a chemoattractant stimulus. Therefore, we started our investigation of impaired neutrophil migration at the level of the initial chemotactic signal. LTB4 acts via the G protein-coupled receptors BLT1 and BLT2. In the mouse, neutrophils express only BLT1, and this receptor is vital to murine experimental arthritis.39,42,43 Signaling via BLT1 and other G protein-coupled receptors is regulated at multiple levels, including surface expression and receptor sensitivity. However, we found no effect of anti-Ly6G preincubation on Ca2+ flux or phosphorylation of ERK or AKT after stimulation with LTB4 (Figure 5A and supplemental Figure 5). Similarly, in our migration assay, LTB4 dose ranging identified no suggestion of receptor desensitization or hypersensitization: pretreatment with anti-Ly6G uniformly suppressed migration, rather than shifting the bell-shaped dose-response curve (Figure 5B).43 These experiments indicate that the inhibitory effect of anti-Ly6G on LTB4-induced neutrophil migration lies downstream of the initial chemotactic signal.
Anti-Ly6G does not change LTB4 receptor sensitivity or LTB4-induced Ca2+ flux. (A) Purified neutrophils were loaded with Fura 2AM for 30 minutes at 37°C. Cells were then washed and pretreated with isotype or anti-Ly6G (10 μg for 10 minutes), and the Ca2+ concentration was monitored continuously with a Hitachi F-4500 calcium fluorometer from 0-200 seconds. LTB4 at 100, 1, or 0.1nM was added 50 seconds after reading. Shown is the isotype (Iso) and anti-Ly6G overlap. (B) BM cells were pretreated with isotype or anti-Ly6G (10 μg for 10 minutes) and neutrophil migration toward LTB4 at a range of concentrations was evaluated after 3 hours using flow cytometry. Results are representative of 2 independent experiments. *P < .05.
Anti-Ly6G does not change LTB4 receptor sensitivity or LTB4-induced Ca2+ flux. (A) Purified neutrophils were loaded with Fura 2AM for 30 minutes at 37°C. Cells were then washed and pretreated with isotype or anti-Ly6G (10 μg for 10 minutes), and the Ca2+ concentration was monitored continuously with a Hitachi F-4500 calcium fluorometer from 0-200 seconds. LTB4 at 100, 1, or 0.1nM was added 50 seconds after reading. Shown is the isotype (Iso) and anti-Ly6G overlap. (B) BM cells were pretreated with isotype or anti-Ly6G (10 μg for 10 minutes) and neutrophil migration toward LTB4 at a range of concentrations was evaluated after 3 hours using flow cytometry. Results are representative of 2 independent experiments. *P < .05.
Anti-Ly6G does not activate neutrophils
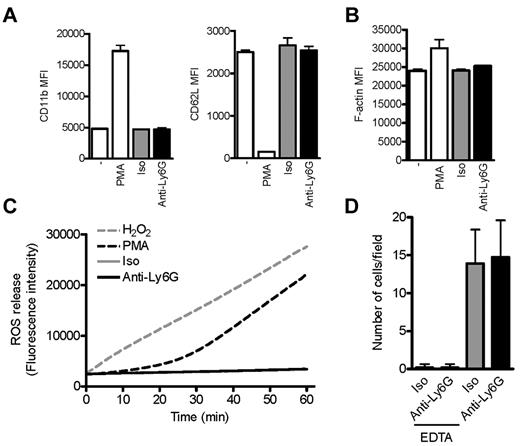
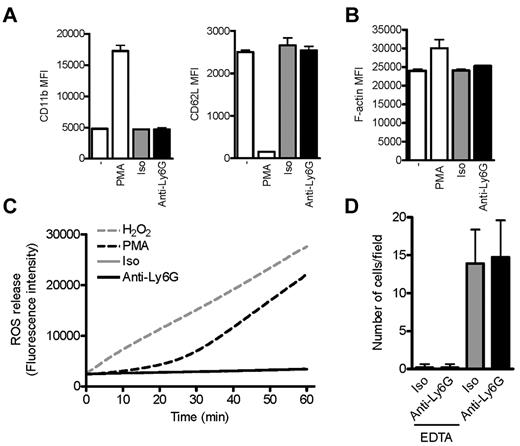
Because neutrophil migration requires physical deformation, we explored whether anti-Ly6G might “preactivate” neutrophils, resulting in enhanced rigidity and therefore failure to cross the transwell membrane. Indeed, cross-linking of anti–Gr-1 F(ab′)2 fragments induces F-actin and CD11b expression, whereas neutrophil activation might explain the lethal outcome of anti-Ly6G administration into TNFα-treated mice.16,17 We therefore used a range of assays to assess neutrophil activation and endothelial adhesion. We found no effect of anti-Ly6G on expression of the activation markers CD11b and CD62L, actin polymerization, generation of ROS, or adherence to TNFα-activated murine endothelium under low physiologic wall shear stress (Figure 6A-D). Therefore, we could not identify direct activation of neutrophils by Ly6G ligation as used in our system.
Ab ligation of Ly6G does not affect markers of neutrophil activation or adhesion to inflamed endothelium. (A) Flow cytometric assessment of activation markers CD11b and CD62L on CD45+Gr-1+CD11bhi cells (10 minutes). (B) F-actin as assessed by phalloidin staining after preincubation with anti-Ly6G, isotype, or PMA (10 minutes). (C) Kinetic staining for ROS. Panels A-C reflect the findings of at least 2 experiments. (D) Adhesion of purified BM neutrophils preincubated with anti-Ly6G or isotype to TNFα-activated bEND5 endothelial cells under low physiologic flow conditions (0.5 dyne/cm2). Shown is abrogation by EDTA consistent with calcium dependence of the neutrophil-endothelium interaction. Data are representative of 3 independent experiments.
Ab ligation of Ly6G does not affect markers of neutrophil activation or adhesion to inflamed endothelium. (A) Flow cytometric assessment of activation markers CD11b and CD62L on CD45+Gr-1+CD11bhi cells (10 minutes). (B) F-actin as assessed by phalloidin staining after preincubation with anti-Ly6G, isotype, or PMA (10 minutes). (C) Kinetic staining for ROS. Panels A-C reflect the findings of at least 2 experiments. (D) Adhesion of purified BM neutrophils preincubated with anti-Ly6G or isotype to TNFα-activated bEND5 endothelial cells under low physiologic flow conditions (0.5 dyne/cm2). Shown is abrogation by EDTA consistent with calcium dependence of the neutrophil-endothelium interaction. Data are representative of 3 independent experiments.
Ly6G associates with β2-integrins
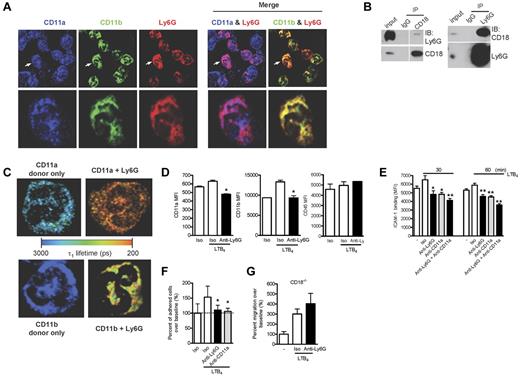
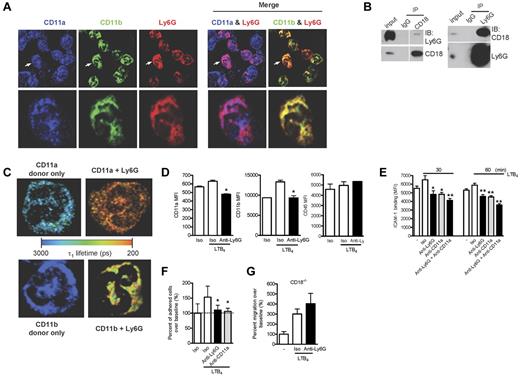
Recent studies on the human neutrophil Ly6/uPAR family member CD177 have identified an association with the β2-integrins CD11a/CD18 (LFA-1) and CD11b/CD18 (Mac-1).14 Considering the possibility that CD177 and Ly6G could be functionally homologous, we examined the distribution of Ly6G and β2-integrins by high-resolution confocal microscopy and found colocalization with both CD11a and CD11b (Figure 7A). Further, after in situ cross-linking, Ly6G and CD18 could reciprocally coimmunoprecipitate (Figure 7B), and we used FLIM to confirm this interaction. As shown in Table 1 and the representative image in Figure 7C, the decrease in τ1 indicates an interaction between Ly6G and CD11a and also between Ly6G and CD11b. This decrease was not seen when we probed the interaction between CD11b and CD45 (Table 1). Interestingly, the percentage of CD11a molecules interacting with Ly6G (a1%) was higher than that seen for CD11b. These data strongly support a direct association of Ly6G with β2-integrins.
Ly6G ligation down-regulates the expression of β2-integrins and neutrophil adhesion and migration via an integrin-dependent mechanism. (A) BM cells were cytospun, fixed, permeabilized, and stained with Abs to CD11a, CD11b, and Ly6G. The images were acquired using confocal microscopy (60× objective). The higher magnification of a representative single-cell image (arrow denotes the cell selected for enlarged viewing) are shown in parallel. The results are representative of at least 3 independent experiments. (B) Coimmunoprecipitation studies were carried out with lysates prepared from BM cells after in situ cross-linking. Ly6G was coimmunoprecipitated with CD18 and CD18 was coimmunoprecipitated with Ly6G. (C) BM cells were cytospun, fixed, permeabilized, and stained with FRET pair as indicated. Shown are representative images from each group displaying interacting τ1 lifetimes in each pixel on a pseudocolor scale (60× objective). (D) Neutrophils were exposed to LTB4 and surface expression of CD11a and CD11b was measured at 60 minutes by flow cytometry. Data are representative of at least 3 independent experiments. (E) Neutrophils were preincubated with anti-Ly6G, anti-CD11a, or the combination at 10 μg/mL for 10 minutes and then with or without LTB4 (100nM) for 30 and 60 minutes. FITC-labeled ICAM-1 binding on neutrophils was detected by flow cytometry. Results are representative of 3 independent experiments. (F) Adhesion of LTB4-activated neutrophils to inflamed endothelium was assessed under high shear stress (10 dyne/cm2) after exposure to anti-Ly6G or control. Data reflect triplicate conditions from 2 independent experiments (CD11a blockade control from 1 experiment). (G) Blockade of transwell migration by anti-Ly6G requires the expression of the β2-integrin CD18. Data are representative of 3 independent experiments. *P < .05; **P < .01 isotype versus anti-Ly6G or anti-CD11a.
Ly6G ligation down-regulates the expression of β2-integrins and neutrophil adhesion and migration via an integrin-dependent mechanism. (A) BM cells were cytospun, fixed, permeabilized, and stained with Abs to CD11a, CD11b, and Ly6G. The images were acquired using confocal microscopy (60× objective). The higher magnification of a representative single-cell image (arrow denotes the cell selected for enlarged viewing) are shown in parallel. The results are representative of at least 3 independent experiments. (B) Coimmunoprecipitation studies were carried out with lysates prepared from BM cells after in situ cross-linking. Ly6G was coimmunoprecipitated with CD18 and CD18 was coimmunoprecipitated with Ly6G. (C) BM cells were cytospun, fixed, permeabilized, and stained with FRET pair as indicated. Shown are representative images from each group displaying interacting τ1 lifetimes in each pixel on a pseudocolor scale (60× objective). (D) Neutrophils were exposed to LTB4 and surface expression of CD11a and CD11b was measured at 60 minutes by flow cytometry. Data are representative of at least 3 independent experiments. (E) Neutrophils were preincubated with anti-Ly6G, anti-CD11a, or the combination at 10 μg/mL for 10 minutes and then with or without LTB4 (100nM) for 30 and 60 minutes. FITC-labeled ICAM-1 binding on neutrophils was detected by flow cytometry. Results are representative of 3 independent experiments. (F) Adhesion of LTB4-activated neutrophils to inflamed endothelium was assessed under high shear stress (10 dyne/cm2) after exposure to anti-Ly6G or control. Data reflect triplicate conditions from 2 independent experiments (CD11a blockade control from 1 experiment). (G) Blockade of transwell migration by anti-Ly6G requires the expression of the β2-integrin CD18. Data are representative of 3 independent experiments. *P < .05; **P < .01 isotype versus anti-Ly6G or anti-CD11a.
Blockade of migration by Ly6G ligation in activated neutrophils is mediated through β2-integrins
Recognizing that β2-integrins, in particular LFA-1, are absolutely required for in K/BxN serum transfer arthritis,44 we investigated whether anti-Ly6G could affect integrin expression and function in activated neutrophils despite the absence of effect in nonactivated cells. We therefore examined neutrophils preincubated with anti-Ly6G or isotype and then exposed to LTB4. After control Ab, LTB4 induced minimal change in CD11a but a marked increase in CD11b expression; in contrast, stimulation after anti-Ly6G decreased surface expression of CD11a and sharply attenuated the increase in surface CD11b (Figure 7D). Whereas expression of CD11a or CD11b remained intact in unstimulated neutrophils exposed to anti-Ly6G (Figure 6A and data not shown), we doubt that this effect reflects steric hindrance of anti-integrin binding. To assess integrin function, we stained LTB4-activated neutrophils with labeled ICAM-1 and observed that pretreatment with anti-Ly6G strongly inhibited LTB4-induced ICAM-1 binding (Figure 7E). The combination of anti-Ly6G and anti-CD11a was slightly more potent than anti-CD11a alone, suggesting that most but not all of this effect was mediated via CD11a (Figure 7E). Consistent with this functional assay, anti-Ly6G aborted firm adhesion to activated endothelium under high shear stress flow conditions, an effect equivalent to that of CD11a blockade (Figure 7F). To determine whether this integrin phenotype was relevant for the effect of anti-Ly6G on migration, we performed our transwell assay in neutrophils lacking β2-integrins (CD18−/−). Interestingly, whereas integrin-independent migration was preserved (excluding a complete block of cell motility), no further effect of anti-Ly6G could be observed (Figure 7G) despite intact expression of Ly6G in CD18−/− neutrophils (Forlow et al45 and data not shown). These studies implicate β2-integrins in the inhibition of migration by Ly6G ligation, providing a compelling explanation for blockade of arthritis in the absence of neutrophil depletion.
Discussion
Neutrophilic infiltration is the sine qua non of inflammatory arthritis. Neutrophil flux through a single inflamed human joint can be as high as 1 × 109 cells/d.46 Murine studies have demonstrated the essential role of these cells in the initiation and propagation of joint inflammation.20-23 In the present study, we investigated the unexpected observation that ligation of Ly6G markedly attenuated K/BxN serum transfer arthritis even without substantial neutrophil depletion. A similar effect was evident in thioglycollate peritonitis. The remarkable absence of neutrophils from joint tissue in treated mice suggested either that recruited neutrophils were rapidly cleared or that recruitment was blocked. Our data strongly suggest the latter, because we found no effect of anti-Ly6G on neutrophil apoptosis, whereas transwell studies showed that anti-Ly6G induced a striking migratory blockade. Further studies demonstrated that Ly6G closely associates with β2-integrins and that anti-Ly6G attenuated both the surface expression and function of β2-integrins. Consistent with the importance of this association with β2-integrins, anti-Ly6G no longer altered migration of CD18−/− neutrophils. These findings identify a previously undefined role for Ly6G as a functional modulator of neutrophil migration and implicate β2-integrins in this activity.
Our observations shed new light on Ly6G, the targeting of which has been a mainstay of experimental murine immunology. We show that the effects of anti–Gr-1 and anti-Ly6G on neutrophil function surpass their impact on circulating neutrophil counts. Our finding that anti-Ly6G abrogates integrin-mediated adherence is consistent with the finding that injection of anti–Gr-1 induces detachment of neutrophils from endothelium in vivo.16 Whereas we did not observe alteration in apoptosis, as was reported by Ribechini et al, this difference may reflect the timing of treatment, the choice of agent (anti–Gr-1 vs anti-Ly6G), or the dose used (250 μg vs 5-10 μg).18
Interestingly, in mice pretreated with TNFα, administration of either anti–Gr-1 or anti-Ly6G promotes development of leukocyte/platelet aggregates, leading to disseminated intravascular coagulation.16,17 Anti–Gr-1–induced adherence between platelets and leukocytes was also found in mice with proteoglycan-induced arthritis, an effect replicated with anti-CD44 and also recombinant P-selectin-Fc fusion protein.47 Neutropenia induced by these agents could be attenuated by heparin or platelet depletion, suggesting that aggregate formation is an important mechanism of Ab-mediated neutrophil depletion.47 Further, a recent study in transfusion-related acute lung injury found that abrogation of disease via anti–Gr-1 was mediated not through neutrophil depletion, but rather by complement consumption; Abs against erythrocytes were just as effective.48 These results highlight the need to remain alert for experimental artifacts when using cell-targeting Abs. However, several lines of evidence suggest that our findings reflect the biology of Ly6G. In vivo, circulating neutrophil counts and morphology remained largely unchanged over the course of arthritis, and anti-Ly6B.2, a neutrophil-binding Ab of matched isotype, failed to attenuate arthritis. In vitro, platelets and complement were not required for either migration or endothelial adherence phenotypes. Therefore, our results most likely reflect specific functions of Ly6G, although further investigation will be required to establish this mechanism in molecular detail.
Careful study of IV anti-Ly6G and anti–Gr-1 has identified transient reductions in circulating neutrophils, even at doses similar to those used in the present study.17,49 Studies using radiolabeled anti–Gr-1 suggest that tagged cells are cleared in the liver and spleen and that neutrophils reconstituting the circulating pool are derived from the BM.49 If this were the case with low-dose anti-Ly6G, then some of its anti-arthritic effect might derive from reduced arthritogenic potency of freshly released BM neutrophils. However, we doubt that replacement represents a major mode of action of low-dose anti-Ly6G. First, the neutropenia we observed after IP Ab administration was less profound than that reported with IV dosing. Second, murine BM and circulating neutrophils exhibit comparable functionality.50 Indeed, adoptively transferred BM neutrophils are quite capable of mediating K/BxN arthritis.3,23,39 Finally, our time-course studies showed that > 93% circulating neutrophils maintained mature morphology after anti-Ly6G treatment. These considerations render the replacement hypothesis unlikely.
Multiple questions remain. Does anti-Ly6G agonize Ly6G, clustering these molecules together to initiate an inhibitory signal? Alternately, does anti-Ly6G antagonize a normal function of Ly6G to promote integrin-mediated neutrophil migration? Extrapolation from related proteins sheds little light on these alternatives. Whereas GPI-linked proteins cannot by themselves initiate intracellular events, they may associate with other proteins to do so. Some Ly6 family members serve to tether a functionally important protein to the cellular surface, as observed with CD177 binding to PR3. These possibilities highlight the need to identify the ligands and surface binding partners of Ly6G, as well as their associated signaling pathways.
Interest in these questions is heightened by similarities between Ly6G and the human neutrophil protein CD177. Our data indicate that Ly6G, like CD177, exhibits both spatial and functional interactions with β2-integrins. Further, Ab ligation of both molecules inhibits neutrophil migration, as demonstrated recently for CD177 using a transendothelial system.15 These observations suggest that Ly6G may possess important functional homology with CD177, potentially providing an opportunity for in vivo experimental manipulation that is currently unavailable for the human protein.
The results of the present study demonstrate a previously unrecognized activity for Ly6G ligation as a modulator of neutrophil migration via a mechanism dependent on β2-integrins. This observation is of interest not only because Ly6G is frequently targeted experimentally, but also because our results imply the existence of a distinct new pathway regulating neutrophil migration in vivo, offering a potential therapeutic opportunity to control the participation of neutrophils in inflammatory disease.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Professor Britta Engelhardt for bEND5 endothelial cells; Dr Haribabu Bodduluri for helpful discussions; Dr Ruben Carrasco for hematopathology expertise; and Drs I-Cheng Ho, Tanya Mayadas-Norton, Paul Monach, and Elaine Remold-O'Donnell for thoughtful comments on the manuscript.
This work was supported by grants from the Arthritis National Research Foundation, the Charles H. Hood Foundation, the Harvard Skin Disease Research Center, and the Cogan Family Foundation (to P.A.N.); the National Science Council, Taiwan (to C.-C.S.); and the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (P30 AR42689 to R.C.F.; 5RO1-AI068871, 3RO1AI068871-04S, 1S10RR027931, and 1R01HL097796 to R.J.S.; and K01 DK089145 to A.B.).
National Institutes of Health
Authorship
Contribution: J.-X.W. designed and performed experiments, analyzed the data, and wrote the manuscript; A.M.B. assisted with the FLIM assay and analyzed the data; S.L.K. assisted with the flow chamber assay and analyzed the data; R.S. and Y.-F.H. assisted with the neutrophil purification, platelet depletion, and cell apoptosis experiments; A.M.B., C.-C.S., R.J.S., and R.C.F. contributed important intellectual insights; P.A.N. conceived and directed the project, performed experiments, analyzed the data, wrote the manuscript, and assumes full responsibility for the integrity and interpretation of the data; and all authors critically reviewed the manuscript and agree to its publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter A. Nigrovic, MD, Brigham and Women's Hospital, Division of Rheumatology, Immunology and Allergy, Smith Bldg, Rm 516c, One Jimmy Fund Way, Boston, MA 02115; e-mail: pnigrovic@partners.org.