Abstract
Although estrogens are known to have a deleterious effect on the venous thrombosis risk and a preventive action on the development of arterial atheroma, their effect on platelet function in vivo remains unclear. Here, we demonstrate that a chronic high physiologic level of estradiol (E2) in mice leads to a marked decrease in platelet responsiveness ex vivo and in vivo compared with ovariectomized controls. E2 treatment led to increased bleeding time and a resistance to thromboembolism. Hematopoietic chimera mice harboring a selective deletion of estrogen receptors (ERs) α or β were used to demonstrate that the effects of E2 were exclusively because of hematopoietic ERα. Within ERα the activation function-1 domain was not required for resistance to thromboembolism, as was previously shown for atheroprotection. This domain is mandatory for E2-mediated reproductive function and suggests that this role is controlled independently. Differential proteomics indicated that E2 treatment modulated the expression of platelet proteins including β1 tubulin and a few other proteins that may impact platelet production and activation. Overall, these data demonstrate a previously unrecognized role for E2 in regulating the platelet proteome and platelet function, and point to new potential antithrombotic and vasculoprotective therapeutic strategies.
Introduction
Estradiol-17β (E2) and estrogen receptors (ERs) are well known for their pivotal role in sexual development and reproduction. However, they can also modulate cardiovascular and metabolic risk, autoimmune disease progression, and cancer growth. These pleiotropic effects are a consequence of both the widespread expression of ER in many cell populations within the body as well as possibly reflecting the ancestral status of ER in the steroid receptor family.1 Epidemiologic and experimental studies now support an atheroprotective effect of both endogenous and exogenous estrogens. The Women's Health Initiative study did not show a coronary protective effect of estrogen in postmenopausal women,2 but subsequent studies have shown that this was because of both inappropriate timing (ie, administering the hormone therapy too late) and the identity of the associated progestin.3,4 The route of administration of estrogens (oral versus transdermal) has also been shown to have a major impact on the risk of venous thromboembolism.5 Together these studies indicate that the beneficial or deleterious action of estrogens are strongly influenced by the dose, route, and timing of the hormonal treatment alongside age, genetic, and environmental factors.
Although the impact of estrogens on coagulation and venous thromboembolism risk has received much attention, their effect on platelet function remains poorly characterized in vivo. After estrogen treatment, one study reported reduced platelet activation6 whereas 2 others found increased activation.7,8 Platelet activation markers were also found at higher levels in postmenopausal compared with premenopausal women.9 Furthermore, platelet aggregation and dense granule secretion were reportedly decreased after E2 treatment in animal models including rabbit and pig.10–13 Although these studies favor an inhibitory role for E2 on platelet activation, there is clearly a need for further investigation.
E2 effects are mediated by ERα and/or ERβ which are members of the nuclear receptor superfamily encoded by 2 distinct genes.14 ERα can be divided into 6 domains, labeled A to F, and harbors 2 transactivation functions (AF-1 and AF-2) which are located within regions A/B and E, respectively.2 Both ERα and β are expressed in megakaryocytes and circulating platelets.15–17 In contrast to the in vivo studies discussed in the previous paragraph,10–13 addition of E2 to washed platelets in vitro was shown to increase the activity of intracellular signaling molecules such as Src, Pyk2, and phosphoinositide 3-kinase through the extragenomic effects of ERβ, leading to a potentiation of platelet activation by subthreshold concentrations of thrombin.18 However, it should be emphasized that a major difference between acute in vitro E2 addition and chronic in vivo E2 treatment is that the latter can have an impact on platelets through genomic mechanisms and can also affect the differentiation of myeloid progenitors19 and megakaryocytes.20 Therefore, the effect of estrogens on platelet function in vivo remains currently unclear.
In this study, we examined the effect of E2 on platelets using a mouse model. Although endogenous estrogens had no significant influence on platelet function, a chronic high physiologic dose of E2 (200 μg/kg/d of E2 injected subcutaneously) decreased platelet responsiveness ex vivo, increased tail-bleeding time, and protected animals against thromboembolism. Using mice in which either ERα or ERβ was inactivated, we found that the hematopoietic ERα alone was responsible for the observed E2 effects. Although ERα AF-1 is mandatory for its reproductive actions, it is dispensable in mediating the atheroprotective properties of E2.21 We found here that AF-1 is also not essential for the effects of E2 on thromboembolism. In addition, we provided evidence that E2 treatment can modulate the platelet proteome. These novel effects of E2 treatment on mouse platelets in vivo suggest its potential for strategies for antiplatelet and vasculoprotective therapies.
Methods
Mice and surgical procedures
Female C57BL/6J mice were purchased from Charles River. ERα-deficient mice (ERα−/−) were maintained at our animal facility and screened by polymerase chain reaction (PCR) genotyping, as previously described.22 ERα+/−, ERβ−/−, and ERα AF-1+/0 mouse founders were generously provided by P. Chambon and A. Krust (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France). Mice were anesthetized by intraperitoneal injection of ketamine (25 mg/kg) and xylazine (10 mg/kg) and ovariectomized or sham-operated at 4 weeks of age and given or not 60-day time-release E2 pellets (0.25 mg E2, releasing on average 200 μg kg−1 d−1 200 μg/kg/d; Innovative Research of America) implanted subcutaneously into the scapular region at 7 weeks of age (except in bone marrow transplantation experiments where it was at 10 weeks of age). They were euthanized after a 3-week E2 treatment period. All procedures were performed in accordance with the recommendations of the European Accreditation of Laboratory Animal Care.
Bone marrow transplantation
Two weeks after ovariectomy, recipient mice were lethally irradiated (9.2 Gy, γ source) then intravenously reconstituted with bone marrow cells from either ERα−/−, ERα+/+, ERβ−/−, or ERβ+/+; AF-1° or AF-1+/+ mice. Two weeks later, mice were implanted or not with an E2 pellet. Bactrim (400 mg/mL sulfamethoxazole, 80 mg/mL trimethoprim) was added to their drinking water for 3 weeks after bone marrow transplantation.
Materials
Collagen was from Nycomed, U46619 was from QBiogene Inc, PPACK was from Calbiochem, and FITC-labeled anti–P-selectin Ab was from BD Biosciences/BD Pharmingen. DiOC6 and Oregon Green 488–conjugated fibrinogen were from Invitrogen. FITC-labeled anti-GPVI and FITC-labeled anti-integrin α2 chain (CD49b) Abs were from Emfret Analytics. Anti-phospho-MLC (Ser19) and anti-total MLC Abs were from Santa Cruz Biotechnology Inc. All other reagents were purchased from Sigma-Aldrich. Convulxin, kindly provided by Dr M. Jandrot-Perrus (Inserm 698, Paris, France), was purified from the venom of Crotalus durissus terrificus.23 The β1 tubulin ELISA kit was from Antibodies-Online and ICI 182780 from Tocris Bioscience.
Tail-bleeding time
Preparation of platelets and in vitro aggregation studies
Platelets were prepared as previously described25 and resuspended in modified HEPES-Tyrode buffer containing 2mM CaCl2 (pH 7.38) at a density of 5 × 108 platelets/mL in the presence of the ADP scavenger apyrase (adenosine-5′-triphosphate diphosphohydrolase) then incubated for 1 hour at 37°C before stimulation. Platelets were counted by microscopy using a Malassez chamber. Optical platelet aggregation experiments were monitored by a turbidimetric method using a dual-channel aggregometer (Payton Associates) with continuous stirring at 900 rev/min, 37°C.
Fibrinogen-binding assays
Washed platelets (1 × 108 platelets/mL) were incubated simultaneously with 0.1 or 0.3 IU/mL thrombin, Oregon Green 488–conjugated fibrinogen (150 μg/mL in final volume) and Tyrode buffer in a final volume of 100 μL. After 10 minutes at 37°C without shaking, samples were fixed by the addition of formalin (1% in final volume) and then diluted 5 times with HEPES-Tyrode buffer. Samples were analyzed by flow cytometry using a FACScan and Win MDI software.
Clot retraction experiments
Platelet-rich plasma (PRP) was obtained from pooled blood samples from several mice by centrifugation for 4 minutes at 250g at 37°C. Platelets were then washed with modified HEPES-Tyrode buffer (pH 6.7) containing 2mM EGTA and 0.35% BSA, and resuspended (3 × 108 platelets/mL) without heparin in their autologous platelet-poor plasma containing 2mM MgCl2 and 2mM EGTA. Thrombin and atroxin were added at a final concentration of 2 IU/mL and 0.1 μg/mL, respectively, and the reaction mixtures were left unstirred at 37°C, as previously described.24,25
Gel electrophoresis and immunoblotting
Proteins were analyzed as previously described25 using the relevant Abs.
Ex vivo flow-based adhesion studies
Glass microcapillaries (Ibidi) were coated with 500 μg/mL type I collagen from equine tendon for 1 hour at 37°C. Bioflux plates (Bioflux 200 from Labtech) were coated with 100 μg/mL fibrinogen. Blood was drawn into lepirudin (200 IU/mL), and DiOC6 (2μM, 30 minutes at 37°C) was used to label platelets in whole blood. Labeled blood was then perfused through collagen-coated glass microcapillaries at a wall shear rate of 1500 seconds−1, followed by washing for 2 minutes at the same shear rate with PBS. For fibrinogen-coated microcapillaries, PPACK-treated whole blood (80μM) was perfused through bioflux plates at a wall shear rate of 1500 seconds−1 or 4000 seconds−1. Thrombus formation was visualized with a 40× long-working-distance objective in real time (acquisition rate: 1 frame every 5 seconds) for both fluorescent and transmitted light microscopy and the analysis was performed as previously described.24,25
Carotid artery thrombosis
The right and left carotids were dissected free from surrounding tissues. Two flow probes were connected to a Transonic model T403 flow meter (Transonic System; Emka Technologies) to record the blood flow (milliliters per minute) of the carotids. Arteries and probes were covered with lubricating jelly and the position of the probe was adjusted. FeCl3 (Mallinckrodt Chemical) was used to induce vascular injury. A 1 × 4-mm strip of paper saturated with 7% FeCl3 solution was applied to the adventitial surface of the right carotid for 2 minutes then removed. The left carotid was used as an internal control. Blood flow was then monitored continuously throughout the procedure (IOX software).25
Thromboembolism
Acute systemic vascular thromboembolism was induced by injecting a mixture of collagen (0.4 mg/kg) and epinephrine (60 μg/kg) into the right jugular vein of anesthetized mice.24
Histochemical analysis of mouse lung
To visualize thrombi in the pulmonary vasculature, anesthetized mice were euthanized 10 minutes after injection of the collagen (0.4 mg/kg) and epinephrine (60 μg/kg) mixture. Lungs were excised and formalin-fixed. Paraffin sections (5-μm thick) were stained with hematoxylin-eosin and analyzed. Platelets were identified with a rabbit anti-αIIb integrin Ab.
Nano-LC-MS/MS and database search analysis
Proteins were digested by incubating each spot excised from 2-dimensional (2D) gels with modified sequencing grade trypsin overnight at 37°C. The resulting peptides were extracted from the gel and resuspended in 5% acetonitrile, 0.05% trifluoroacetic acid. The peptide mixtures were analyzed by nano-LC-MS/MS using an Ultimate 3000 system (Dionex) coupled to an LTQ-Orbitrap XL mass spectrometer (Thermo Fisher Scientific). The Mascot Daemon software (Version 2.2.0.3; Matrix Science) was used to perform searches in the SwissProt-Trembl database. Mascot results were parsed with the in-house developed software Mascot File Parsing and Quantification (MFPaQ; Version 4.0)26 and an identified protein was considered as a hit if it was identified with at least 2 peptides with a score greater than the significance threshold score for a probability of P < .05 or at least 1 peptide with a score greater than the significance threshold score of P < .001, as determined by the Mascot Search program.
Electron microscopy
Platelets were prepared as previously described,27 then examined using a transmission electron microscope at an accelerating voltage of 5 kV.
Megakaryocyte purification and culture
Mice were ovariectomized and treated or not with E2 under the same protocol as described in “Mice and surgical procedures.” Bone marrow cells were obtained from femora and tibiae of mice by flushing. Cells expressing 1 or more of the following surface proteins, CD16/CD32+, Gr1+, B220+, CD11b+ were depleted using immunomagnetic beads (sheep anti-rat IgG Dynabeads; Invitrogen). The remaining population was cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (containing estrogens), 2mM L-glutamine, penicillin/streptomycin, and 20 ng/mL murine stem cell factor (SCF) at 37°C under 5% CO2 for 2 days in the presence or absence of the anti-estrogen ICI 182780 (10−6M). Cells were then cultured for an additional 4 to 5 days in the presence of 20 ng/mL murine SCF and 100 ng/mL murine thrombopoietin. The cell population was then enriched in mature megakaryocytes using a 1.5%/3% bovine serum albumin (BSA) gradient under gravity (1g) for 45 minutes at room temperature. Megakaryocytes were lysed and their β1 tubulin content was analyzed using the ELISA test.
Statistics
Results are expressed as mean ± SEM. Statistical analyses were performed using Excel software (Student t test). P values less than .05 were considered statistically significant.
Results
Increased bleeding time and platelet aggregation defects after chronic E2 treatment in mice
Ovariectomized mice were treated or not with an E2 pellet for 3 weeks. This type of treatment was previously reported by our team to induce a stable E2 plasma level of approximately 0.3nM (80 pg/mL).28,29 This level is in the high range of pregnant mice.30,31 The average plasma concentration in E2 is below the threshold of detection in untreated ovariectomized mice.
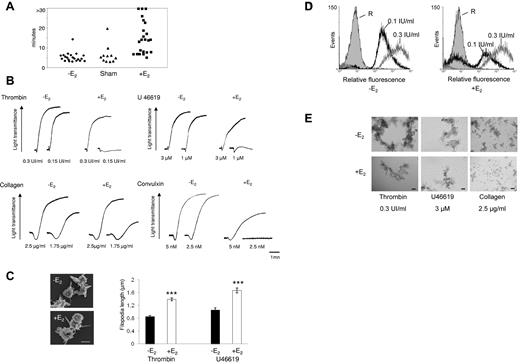
Platelet hemostatic function was analyzed in vivo by measuring tail-bleeding time (Figure 1A). In both control untreated ovariectomized mice producing very low levels of estrogens and sham-operated mice, a normal bleeding time was observed (324 ± 53 seconds and 366 ± 64 seconds, respectively; mean ± SEM), indicating that endogenous E2 levels had no impact on primary hemostasis. Conversely, bleeding times were increased in E2-treated mice: of 23 mice tested, bleeding was weakly prolonged in 9 cases and considerably increased in 14 with 3 of these displaying a bleeding time greater than 30 minutes.
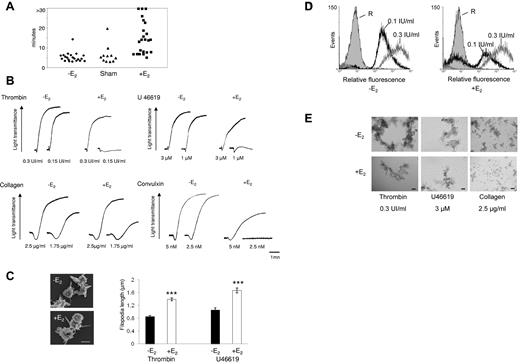
Increased bleeding time and aggregation defects in platelets from E2-treated mice. (A) Tail bleeding times of ovariectomized (-E2), sham-operated (Sham), and ovariectomized E2-treated mice (+E2) were monitored. The experiment was stopped after 30 minutes if no cessation of blood flow occurred (> 30). Each point represents 1 individual. (B) Aggregation of washed platelets from ovariectomized mice treated or not with E2 was initiated by the addition of thrombin, the thromboxane A2 analog U46619, collagen, or the GPVI agonist convulxin and assessed with a chrono-log dual-channel aggregometer with stirring at 900 rev/min. Tracings are representative of at least 5 independent experiments. (C) Scanning electron micrographs of platelets from ovariectomized mice treated or not with E2 and stimulated by thrombin in suspension and under nonaggregating conditions (scale bar: 1 μm). Filopodia length was measured using ImageJ software from scanning electron micrographs. Platelets from E2-treated mice stimulated by thrombin (0.3 IU/mL) or U46619 (3μM) exhibit longer filopodia than platelets from ovariectomized mice. Results are mean values ± SEM from 3 independent experiments and at least 75 platelets. ***Significant difference (P < .001). (D) The binding of labeled fibrinogen to platelets from ovariectomized mice treated or not with E2 and activated by thrombin (0.1 and 0.3 IU/mL) for 10 minutes was measured by flow cytometry. A representative example of 3 independent experiments is shown. R indicates resting. (E) Platelets were activated as described in panel B then fixed with 1.5% paraformaldehyde for 30 minutes at room temperature and observed using differential interference contrast with a Nikon Eclipse TE 2000-U equipped with a 10× objective and a DXM 1200 digital camera (scale bar: 40 μm).
Increased bleeding time and aggregation defects in platelets from E2-treated mice. (A) Tail bleeding times of ovariectomized (-E2), sham-operated (Sham), and ovariectomized E2-treated mice (+E2) were monitored. The experiment was stopped after 30 minutes if no cessation of blood flow occurred (> 30). Each point represents 1 individual. (B) Aggregation of washed platelets from ovariectomized mice treated or not with E2 was initiated by the addition of thrombin, the thromboxane A2 analog U46619, collagen, or the GPVI agonist convulxin and assessed with a chrono-log dual-channel aggregometer with stirring at 900 rev/min. Tracings are representative of at least 5 independent experiments. (C) Scanning electron micrographs of platelets from ovariectomized mice treated or not with E2 and stimulated by thrombin in suspension and under nonaggregating conditions (scale bar: 1 μm). Filopodia length was measured using ImageJ software from scanning electron micrographs. Platelets from E2-treated mice stimulated by thrombin (0.3 IU/mL) or U46619 (3μM) exhibit longer filopodia than platelets from ovariectomized mice. Results are mean values ± SEM from 3 independent experiments and at least 75 platelets. ***Significant difference (P < .001). (D) The binding of labeled fibrinogen to platelets from ovariectomized mice treated or not with E2 and activated by thrombin (0.1 and 0.3 IU/mL) for 10 minutes was measured by flow cytometry. A representative example of 3 independent experiments is shown. R indicates resting. (E) Platelets were activated as described in panel B then fixed with 1.5% paraformaldehyde for 30 minutes at room temperature and observed using differential interference contrast with a Nikon Eclipse TE 2000-U equipped with a 10× objective and a DXM 1200 digital camera (scale bar: 40 μm).
To investigate intrinsic platelet functional defects, aggregation of washed platelets was monitored after stimulation by thrombin and the thromboxane A2 analog (U46619), which both signal via G protein–coupled receptors (Figure 1B). In contrast to ovariectomized mice platelets, which aggregated in a dose-dependent manner to both agonists, platelets from E2-treated mice failed to aggregate in response to low concentrations of thrombin or U46619. At higher concentrations of these agonists, platelets aggregated with only mild defects in intensity and/or velocity. The expression of P-selectin in E2-treated mouse platelets was not modified at high or low doses of thrombin or U46619 (not shown), indicating that α granule secretion was not affected. Platelet shape change was also not modified by these agonists but, using scanning electron microscopy, we consistently observed an increase in the length of filopodia in platelets from E2-treated mice stimulated by thrombin or U46619 in suspension and under nonaggregating conditions (Figure 1C).
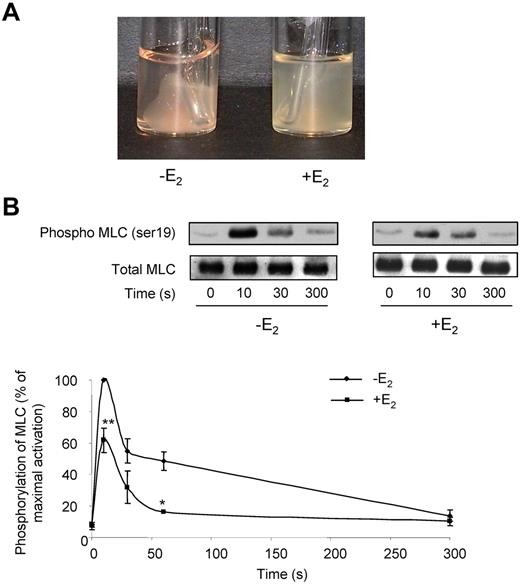
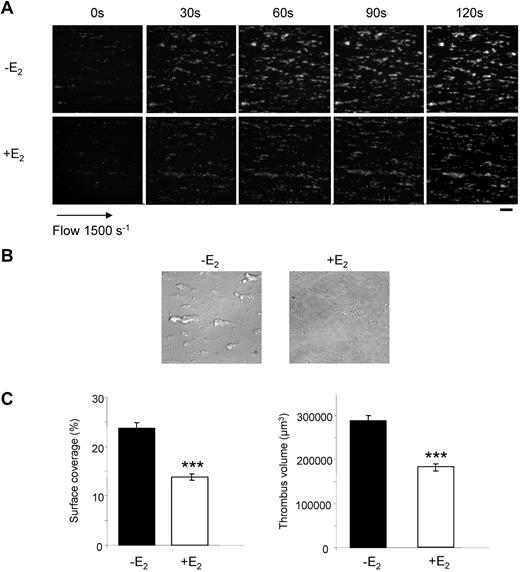
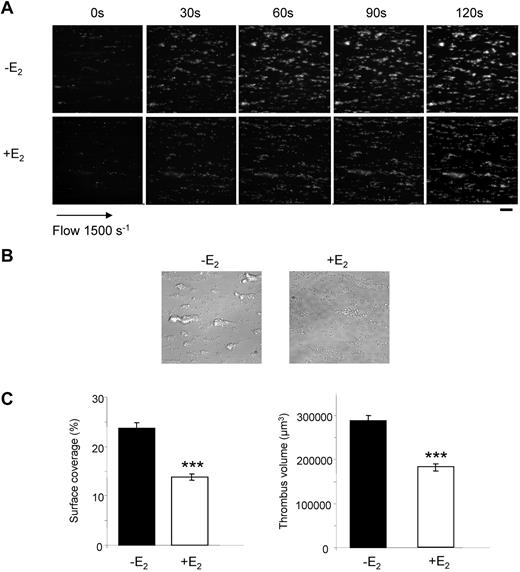
At low doses of collagen, platelets from E2-treated mice aggregated slower and less efficiently than control platelets. Collagen binds to both the GPVI and integrin α2β1 receptors,32 while the snake venom toxin convulxin (Cvx) is a selective GPVI agonist. Platelets from E2-treated mice were much less responsive to 5nM Cvx compared with control platelets and did not aggregate at all at low doses of Cvx (Figure 1B). It is noteworthy that expression of GPVI and α2β1 was not modified by E2 treatment as confirmed by flow cytometry (supplemental Figure 1; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Flow cytometry was also used to assess fibrinogen binding to the αIIbβ3 integrin downstream of thrombin stimulation. In accordance with the aggregation responses, E2 treatment induced a reduction of fibrinogen binding at low thrombin concentrations but not at high concentrations (Figure 1D). However, despite normal fibrinogen binding and aggregation traces in response to high concentrations of agonists, microscopy analysis (Figure 1E) indicated that, compared with controls, platelets from E2-treated mice formed smaller aggregates in response to all agonists tested even at high concentrations. This suggests a defect in αIIbβ3 outside-in signaling. Consistent with this, retraction of the fibrin clot by washed platelets, which also requires functional αIIbβ3 alongside tight membrane-cytoskeleton interactions and the contractile actomyosin system, was strongly reduced on E2 treatment (Figure 2A). In addition, thrombin-induced myosin light chain (MLC) phosphorylation, mandatory for actomyosin cross-bridge formation and contractility, was significantly decreased in platelets from E2-treated mice (Figure 2B). Together, these data suggest a defect in αIIbβ3-mediated platelet responses after E2 treatment. This was confirmed under physiologic arterial flow conditions using a flow-based adhesion assay where whole blood was perfused over a fibrinogen matrix. E2 treatment reduced platelet binding (supplemental Figure 2). We then perfused whole blood over a collagen matrix and analyzed the effects of E2 treatment. After 2 minutes, control mouse platelets formed numerous densely packed thrombi while E2 treatment reduced both the surface coverage and the volume of the thrombi (Figure 3A-C). In addition, platelets from E2-treated mice only formed a single platelet layer in many areas of the collagen matrix. The reduced thrombus volume seen after 2 minutes in E2-treated mice is not thought to be because of the 25% reduction in platelet count observed after E2 (Table 1) because such a reduction did not affect the final thrombus size in control blood under these conditions (M.-P.G., unpublished observation, January 2011).
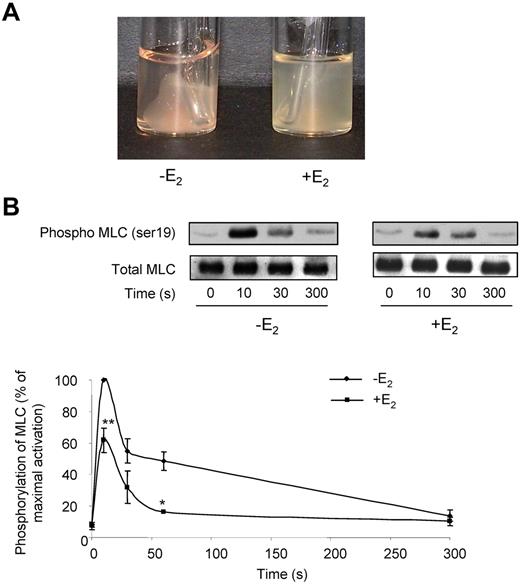
Defects in fibrin clot retraction and MLC phosphorylation in E2-treated mice. (A) Fibrin clot retraction assays were performed in PRP adjusted to 3 × 108 platelets/mL and treated with both thrombin (2 IU/mL) and atroxin (0.1 μg/mL). Photographs shown are representative of 4 independent experiments. (B) Impact of E2 treatment on MLC phosphorylation over time. Platelets from mice treated or not with E2 were stimulated by 0.3 IU/mL thrombin for the indicated time and probed by immunoblotting with an anti-phospho-MLC Ab (ser 19) or a total MLC Ab. Phosphorylation levels were estimated by densitometry analysis of Western blots, and the results are expressed as percentages of maximum phosphorylation (10 seconds stimulation of control mice) and are mean ± SEM of 4 independent experiments. Significant difference **P < .01, *P < .05, according to a Student t test.
Defects in fibrin clot retraction and MLC phosphorylation in E2-treated mice. (A) Fibrin clot retraction assays were performed in PRP adjusted to 3 × 108 platelets/mL and treated with both thrombin (2 IU/mL) and atroxin (0.1 μg/mL). Photographs shown are representative of 4 independent experiments. (B) Impact of E2 treatment on MLC phosphorylation over time. Platelets from mice treated or not with E2 were stimulated by 0.3 IU/mL thrombin for the indicated time and probed by immunoblotting with an anti-phospho-MLC Ab (ser 19) or a total MLC Ab. Phosphorylation levels were estimated by densitometry analysis of Western blots, and the results are expressed as percentages of maximum phosphorylation (10 seconds stimulation of control mice) and are mean ± SEM of 4 independent experiments. Significant difference **P < .01, *P < .05, according to a Student t test.
Thrombus formation defect in E2-treated mice. DiOC6-labeled platelets in whole blood were perfused over a collagen-coated surface at a wall shear rate of 1500 seconds−1 for 2 minutes. (A) Thrombus formation was visualized with a 40× long working distance objective in real time and then imaged by transmitted light microscopy (scale bar: 20 μm). (B) Representative phase contrast images taken at the end of the experiment. (C) Surface area covered by thrombi and thrombus volume were measured at 2 surface locations. Results are mean values ± SEM from 4 independent experiments. ***Significant difference (P < .001).
Thrombus formation defect in E2-treated mice. DiOC6-labeled platelets in whole blood were perfused over a collagen-coated surface at a wall shear rate of 1500 seconds−1 for 2 minutes. (A) Thrombus formation was visualized with a 40× long working distance objective in real time and then imaged by transmitted light microscopy (scale bar: 20 μm). (B) Representative phase contrast images taken at the end of the experiment. (C) Surface area covered by thrombi and thrombus volume were measured at 2 surface locations. Results are mean values ± SEM from 4 independent experiments. ***Significant difference (P < .001).
Although the agonists used in these assays signal via different intracellular pathways, a critical common negative regulator of platelet function is cAMP, therefore cAMP levels were tested in E2-treated platelets. E2 treatment was found to have no effect on cAMP levels neither under resting conditions nor after incubation with PGE1 (supplemental Figure 3).
Overall, our results point to a decrease in platelet responsiveness after long-term E2 treatment in mice. This was observed downstream of numerous agonists that act via different signaling pathways and was consistent across various platelet function assays.
Chronic E2 treatment protects mice from thromboembolism
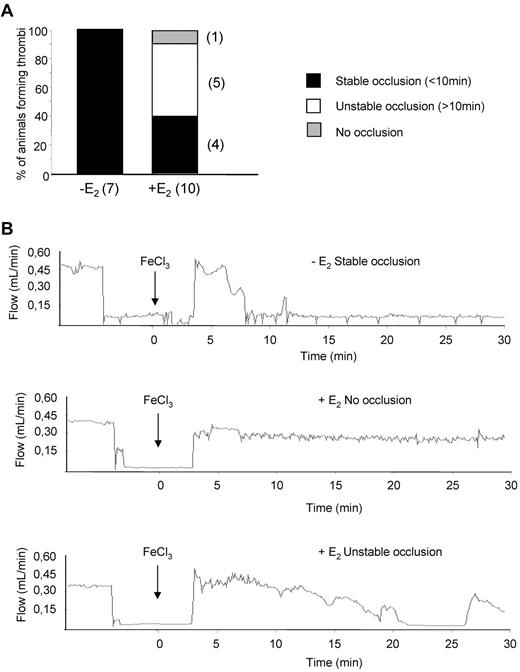
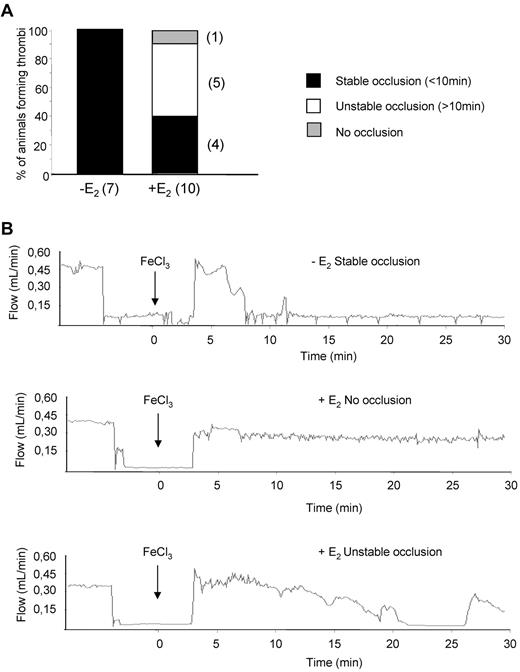
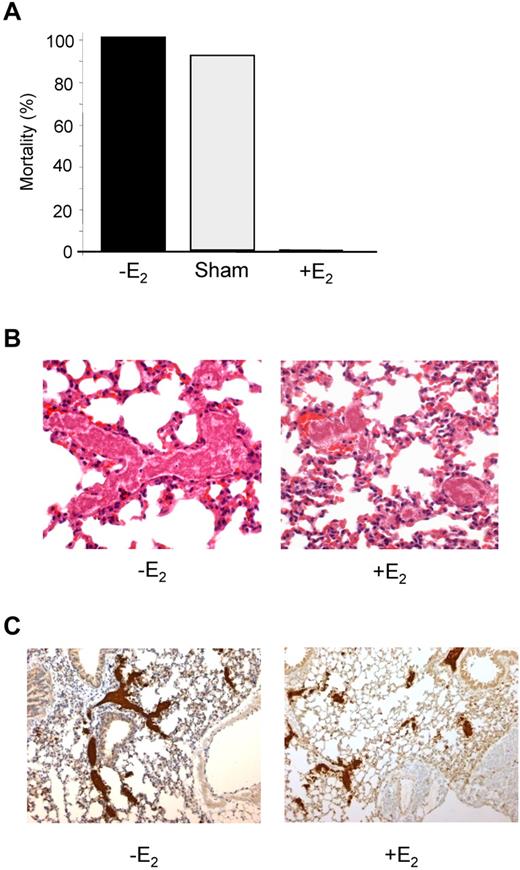
To evaluate the effect of E2 treatment on intravascular platelet aggregation, an injury to the carotid artery was induced by FeCl3 and the velocity of blood flow and time to occlusion were determined. While 100% of control animals presented a complete occlusion within 10 minutes, E2 treatment reduced the formation of a stable occlusive thrombus to 40% of cases (Figure 4). Unstable occlusions were observed in 50% of cases and 10% of the treated mice exhibited a complete resistance to occlusion. The potential of E2 in preventing occlusive thrombus formation in vivo was tested using another model of acute vascular occlusion that was induced by intrajugular injection of a mixture of collagen (0.4 mg/kg) and epinephrine (60 μg/kg). As shown in Figure 5A, 100% (12 of 12) of control mice and 91.6% (11 of 12) of sham-operated mice died within 5 minutes of injection. In sharp contrast, 100% (10 of 10) of E2-treated mice survived. Interestingly, these mice were still protected from thromboembolism when a dose of 1 mg/kg collagen was injected (not shown). Histologic examination of lung tissue from control mice revealed large occlusive platelet thrombi throughout the pulmonary vasculature, particularly in large vessels (Figure 5B). Occlusive pulmonary thrombi in E2-treated mice were only observed in small vessels. These thrombi contained significantly less platelets compared with untreated mice, as shown by the intensity of the αIIb integrin staining (Figure 5C). Interestingly, E2 treatment did not modify standard coagulation tests (prothrombin time and activated partial thromboplastin time), although it increased levels of plasma fibrinogen and factors VIII, IX, and XI (supplemental Table 1). Overall, these results clearly indicate that, after E2 treatment, mice are protected from intravascular thrombosis independent of functional deficiencies in coagulation.
Thrombotic response of mice to ferric chloride injury of the carotid artery. Flow rates were measured in the carotid artery after exposure to 7% FeCl3 for 2 minutes. The experiment was stopped after 30 minutes. (A) For each genotype, the number of mice forming a stable occlusion is shown in black. The number of mice that formed an unstable or partial occlusion is shown in white. The number of mice that did not form an occlusion is shown in gray. (B) Representative flow traces for each case (stable occlusion, no occlusion, and unstable occlusion).
Thrombotic response of mice to ferric chloride injury of the carotid artery. Flow rates were measured in the carotid artery after exposure to 7% FeCl3 for 2 minutes. The experiment was stopped after 30 minutes. (A) For each genotype, the number of mice forming a stable occlusion is shown in black. The number of mice that formed an unstable or partial occlusion is shown in white. The number of mice that did not form an occlusion is shown in gray. (B) Representative flow traces for each case (stable occlusion, no occlusion, and unstable occlusion).
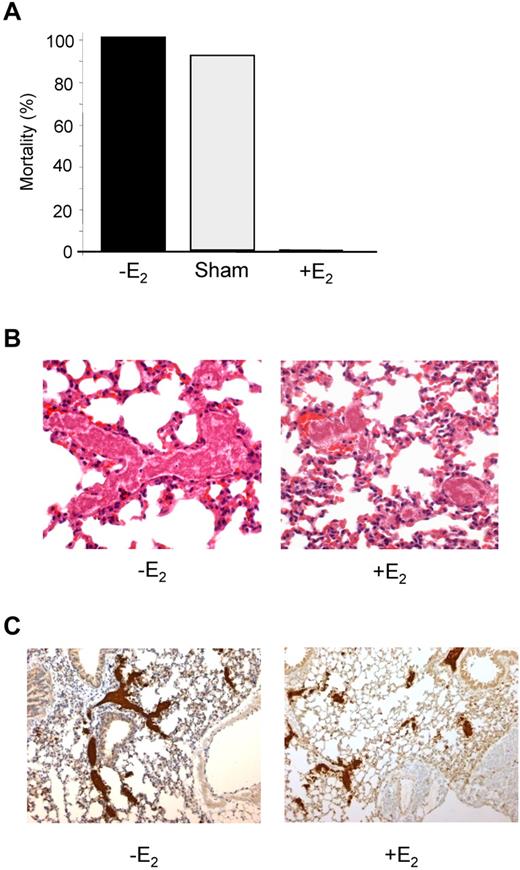
E2-treated mice are protected from collagen/epinephrine-induced thromboembolism. (A) Thromboembolism was induced by injection of a collagen (0.4 mg/kg) and epinephrine (60 μg/kg) mixture into the jugular vein. All non-E2–treated mice (12 of 12) and 11 of 12 sham-operated mice died within 5 minutes, whereas all E2-treated mice (10 of 10) survived. (B) Representative sections of hematoxylin-eosin–stained lungs from a control mouse that died during the assay and an E2-treated mouse that survived and was killed 10 minutes after injection of collagen/epinephrine mixture. Original magnification ×400. (C) Platelet staining (αIIb in brown) of lung sections from mice treated or not with E2. Original magnification, ×100.
E2-treated mice are protected from collagen/epinephrine-induced thromboembolism. (A) Thromboembolism was induced by injection of a collagen (0.4 mg/kg) and epinephrine (60 μg/kg) mixture into the jugular vein. All non-E2–treated mice (12 of 12) and 11 of 12 sham-operated mice died within 5 minutes, whereas all E2-treated mice (10 of 10) survived. (B) Representative sections of hematoxylin-eosin–stained lungs from a control mouse that died during the assay and an E2-treated mouse that survived and was killed 10 minutes after injection of collagen/epinephrine mixture. Original magnification ×400. (C) Platelet staining (αIIb in brown) of lung sections from mice treated or not with E2. Original magnification, ×100.
ERα in hematopoietic cells supports the increased bleeding time and protection from thromboembolism in E2-treated mice
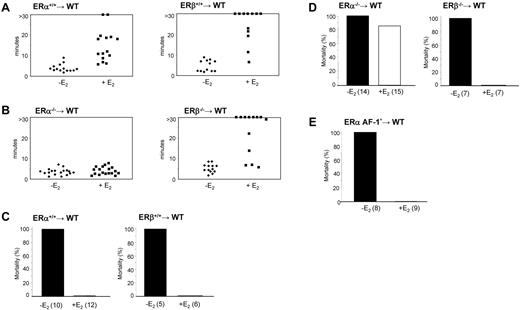
The molecular basis of these E2 effects were investigated by grafting lethally irradiated wild-type ovariectomized mice with bone marrow harvested from ERα+/+, ERβ+/+, ERα−/−, or ERβ−/− mice. PCR was used to confirm the loss of expression of hematopoietic ERα or ERβ in spleen cell lysates from these chimera mice (not shown). Platelet count was not significantly affected by the graft (Table 1). As expected, the tail-bleeding time of most control mice engrafted with wild-type bone marrow was prolonged after E2 treatment (Figure 6A). Interestingly, ERα−/− bone marrow chimeras treated with E2 had a normal bleeding time, whereas that of E2-treated ERβ−/− bone marrow chimeras was similar to control animals, with 7 of 13 mice bleeding for more than 30 minutes (Figure 6B). Thus, hematopoietic ERα, but not ERβ, is required for the prolongation of bleeding time induced by chronic E2 treatment.
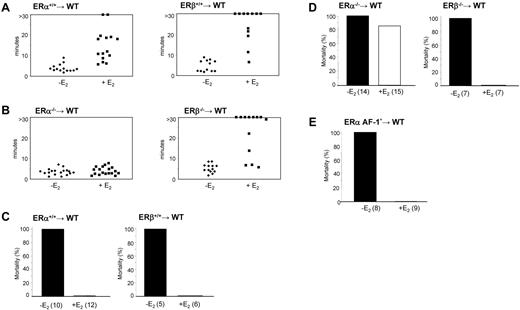
Bleeding time and thromboembolism in ERα, ERβ, or ERα AF-10 hematopoietic chimeric mice. (A) Tail-bleeding times of control mice (WT) engrafted with normal bone marrow (ERα+/+ and ERβ+/+) and treated or not with E2. (B) Tail-bleeding times of ERα−/− and ERβ−/− bone marrow chimeras treated or not with E2. (C) Mice engrafted with normal bone marrow or (D) with bone marrow from ERα−/− or ERβ−/− mice were treated or not with E2 and thromboembolism assays were performed as in Figure 5. Fourteen of 15 mice engrafted with ERα−/− bone marrow died whereas all (7 of 7) mice engrafted with ERβ−/− survived. (E) Thromboembolism assays were performed in mice engrafted with ERα AF-10 bone marrow and treated or not with E2. All non-E2–treated mice (8 of 8) died, whereas all E2-treated mice (9 of 9) survived.
Bleeding time and thromboembolism in ERα, ERβ, or ERα AF-10 hematopoietic chimeric mice. (A) Tail-bleeding times of control mice (WT) engrafted with normal bone marrow (ERα+/+ and ERβ+/+) and treated or not with E2. (B) Tail-bleeding times of ERα−/− and ERβ−/− bone marrow chimeras treated or not with E2. (C) Mice engrafted with normal bone marrow or (D) with bone marrow from ERα−/− or ERβ−/− mice were treated or not with E2 and thromboembolism assays were performed as in Figure 5. Fourteen of 15 mice engrafted with ERα−/− bone marrow died whereas all (7 of 7) mice engrafted with ERβ−/− survived. (E) Thromboembolism assays were performed in mice engrafted with ERα AF-10 bone marrow and treated or not with E2. All non-E2–treated mice (8 of 8) died, whereas all E2-treated mice (9 of 9) survived.
Furthermore, hematopoietic ERα was found to be essential for the E2-mediated protection of thromboembolism observed after injection of a collagen/epinephrine mixture in vivo. Most E2-treated mice reconstituted with ERα−/− bone marrow (86.6%; 13 of 15) died within 5 minutes (Figure 6C-D) while the protective effect of E2 was present in ERα+/+, ERβ+/+, and ERβ−/− bone marrow chimeras. Histologic analysis of lung tissue from these mice confirmed occlusive platelet-rich thrombi localized in both small and large vessels (data not shown).
To study the role of the AF-1 domain of ERα in vivo, we used a mouse model lacking the AF-1 region (ERaAF-10) because of a deletion of the first exon encoding the A/B domain. This resulted in the 66-kDa ERα protein being replaced by a 49-kDa ERα isoform.21 As shown by the generation of hematopoietic chimera, we found that E2-mediated resistance to thromboembolism still occurred in the absence of ERαAF-1 (Figure 6E). Tail-bleeding time also increased on E2 treatment (510 ± 80 seconds vs 250 ± 90 seconds, P < .01, n = 9 and n = 8, respectively), although this effect was not as pronounced as in control E2-treated mice, suggesting a partial contribution of the AF-1 domain.
E2 treatment impacts on the platelet proteome: the example of β1 tubulin
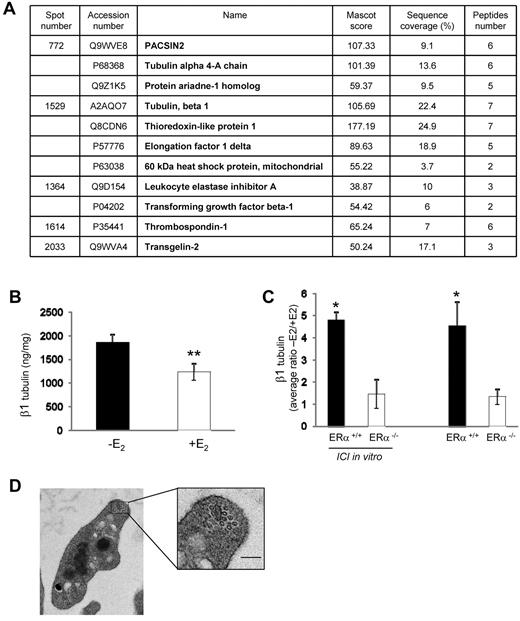
Because E2 treatment could affect megakaryocyte gene expression, we performed differential proteomic analysis using 2D-DIGE coupled to mass spectrometry on platelets from mice treated or not with E2. Results were consistent over 3 independent series (each containing 3 or 4 mice per group): 4 protein spots were found to be down-regulated (1.66-, 1.59-, 1.74-, and 1.73-fold) and 1 was up-regulated (1.99-fold) in E2-treated mice compared with control animals (supplemental Figure 4A-B). Proteins present in these spots were identified by mass spectrometry (Figure 7A) as cytoskeleton proteins (β1 tubulin, α4-A tubulin, and pacsin 2), secreted proteins (transforming growth factor β-1, thrombospondin-1, and leukocyte elastase inhibitor), a protein involved in the regulation of oxidative stress (thioredoxin-like protein), heat shock protein 60, and the elongation factor 1δ. It is noteworthy that a discrepancy between the position on the 2D-DIGE protein spot patterns and the molecular weight or pI was observed for transforming growth factor β-1, thrombospondin-1, and transgelin-2, suggesting that they were in fact fragments of the native proteins. We focused our attention on β1 tubulin which is specifically expressed in platelets and mature megakaryocytes and plays a role in platelet biogenesis, structure, and function.33,34 The role of the other identified proteins is unknown or poorly characterized in platelets. The decreased expression of β1 tubulin in platelets from E2-treated mice observed by 2D-DIGE was confirmed using a specific ELISA test (Figure 7B). Interestingly, the changes observed in platelet proteins appear to originate from initial effects on hematopoietic progenitors. Indeed, the effect of in vivo E2 treatment, through the ERα receptors, was critical for the modulation of β1 tubulin expression in megakaryocytes (Figure 7C). The presence of the anti-estrogen ICI 182780 during the in vitro differentiation did not impact these changes, showing the importance of the in vivo conditioning of the cells. However, as shown by transmission electron microscopy (Figure 7D), the decrease in β1 tubulin had no significant effect on the number of microtubule coils in the marginal band (10.25 ± 0.40 per control platelet vs 9.47 ± 0.54 in E2-treated platelets; n = 27 and n = 17, respectively) nor on the discoid shape and size of the resting platelets (not shown). As shown in Figure 1C, after thrombin or U46619 stimulation in suspension and under nonaggregating conditions, E2-treated platelets extended their filopodia significantly more than control platelets while their hyalomer surface area was higher (2.21 μm2 ± 0.06 vs 1.9 μm2 ± 0.05; P < .01, n = 90 and n = 73, respectively, on thrombin stimulation), suggesting a decrease in internal platelet contraction, a mechanism linked to microtubule reorganization.35
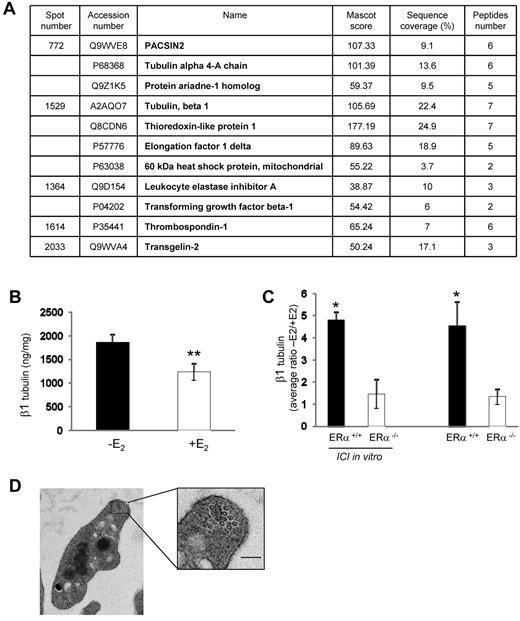
E2 treatment impacts on the platelet proteome. (A) Platelets from 3 or 4 mice treated or not with E2 were pooled, and proteins were labeled with CyDye DIGE Fluor Minimal Dyes as described in supplemental Methods. Results from mass spectrometry analysis are summarized in a table showing accession numbers, names, Mascot score, sequence coverage, and number of peptides for each of the 5 excised spots. The experiment was repeated 3 times. Spots displaying a ≥ 1.5 increase or decrease in abundance with a P value < .05 were selected for protein identification. (B) Level of β1 tubulin in platelets from ovariectomized mice treated or not with E2 (n = 7 and n = 8, respectively) quantified by a specific ELISA test. Results are mean values ± SEM. **Significant difference (P < .01). (C) Average ratios (−E2/+E2) of β1 tubulin expression in megakaryocytes from mice ERα+/+ or ERα−/−. Progenitor cells isolated from bone marrow of mice treated with E2 (n = 4 in each group) or not (n = 3 in each group) were incubated or not with the anti-estrogen ICI 182780 (10−6M) along the differentiation process. Four or 5 days after addition of thrombopoietin (100 ng/mL), Megakaryocytes (MKs) were lysed and their β1 tubulin content was analyzed using the ELISA test. *Significant difference (P < .1) between mice treated or not with E2 in each group (ERα+/+ or ERα−/−). (D) Representative transmission electron microscopy images of a resting platelet from E2-treated mice showing the microtubule coils in the marginal band (scale bar: 0.1 μm).
E2 treatment impacts on the platelet proteome. (A) Platelets from 3 or 4 mice treated or not with E2 were pooled, and proteins were labeled with CyDye DIGE Fluor Minimal Dyes as described in supplemental Methods. Results from mass spectrometry analysis are summarized in a table showing accession numbers, names, Mascot score, sequence coverage, and number of peptides for each of the 5 excised spots. The experiment was repeated 3 times. Spots displaying a ≥ 1.5 increase or decrease in abundance with a P value < .05 were selected for protein identification. (B) Level of β1 tubulin in platelets from ovariectomized mice treated or not with E2 (n = 7 and n = 8, respectively) quantified by a specific ELISA test. Results are mean values ± SEM. **Significant difference (P < .01). (C) Average ratios (−E2/+E2) of β1 tubulin expression in megakaryocytes from mice ERα+/+ or ERα−/−. Progenitor cells isolated from bone marrow of mice treated with E2 (n = 4 in each group) or not (n = 3 in each group) were incubated or not with the anti-estrogen ICI 182780 (10−6M) along the differentiation process. Four or 5 days after addition of thrombopoietin (100 ng/mL), Megakaryocytes (MKs) were lysed and their β1 tubulin content was analyzed using the ELISA test. *Significant difference (P < .1) between mice treated or not with E2 in each group (ERα+/+ or ERα−/−). (D) Representative transmission electron microscopy images of a resting platelet from E2-treated mice showing the microtubule coils in the marginal band (scale bar: 0.1 μm).
Discussion
Using a mouse model alongside ex vivo and in vivo approaches, we found that a chronic high physiologic level of estrogen equivalent to that observed in pregnant mice had a significant inhibitory effect on platelet aggregation. This effect was most obvious when washed platelet aggregation was monitored in response to low concentrations of physiologic agonists but was also detected in response to high concentrations because platelets still formed smaller aggregates. The inhibitory effect of E2 treatment on platelet responsiveness was observed under different conditions and with various agonists known to activate distinct signaling pathways (via either ITAM/tyrosine kinase-linked receptors, heterotrimeric G protein–coupled receptors and/or integrin-linked focal adhesion structures), suggesting that E2 affects a central mechanism controlling the global sensitivity of platelets. The defect in aggregation at low doses of agonists correlated with a decrease in fibrinogen binding to αIIbβ3. At high concentrations of agonists, fibrinogen binding was virtually normal despite a reduction in the size of platelet aggregates. This was because of a decrease in αIIbβ3-mediated functions, as shown by the reduction in platelet-induced fibrin clot retraction and adhesion of platelets to a fibrinogen-coated surface under flow conditions. In vitro thrombus formation assays conducted under physiologic flow conditions on a collagen matrix confirmed that platelets from E2-treated mice are less efficient at forming thrombi compared with control platelets.
Importantly, E2 treatment also has a marked inhibitory effect on platelet activation in vivo. Besides increasing the tail-bleeding time, it induced an impressive resistance to thromboembolism after injection of an epinephrine-collagen mixture and a significant protection to occlusive arterial thrombosis induced by an FeCl3 injury of the carotid. Histologic analysis of mouse lungs after injection of an epinephrine-collagen mixture indicated that both small and large vessels were totally occluded in untreated mice while occlusive thrombi were observed only in small vessels after E2 treatment. E2 treatment did not modify the performance of the coagulation cascade as assessed by standard coagulation tests but it did increase the concentration of fibrinogen and factors VIII, IX, and XI (supplemental Table 1), as previously observed.36 Thus, the resistance to thromboembolism was not because of a decrease in coagulation factor expression and function but was linked to reduced platelet performance.
Hematopoietic chimera mice allowed us to demonstrate that hematopoietic cell expression of ERα, but not ERβ, is mandatory in eliciting the effects of chronic E2 treatment on platelet function. To delineate the role of AF1, we recently generated mice selectively deficient in ERα AF-1. Using these ERαAF-10 mice, we found that ERαAF-1 is indeed dispensable for the major vasculoprotective actions of E2 such as the prevention of atheroma, acceleration of endothelial healing, and increased endothelial nitric oxide production.21 Here, we show that AF-1 is not required for E2-induced resistance to thromboembolism.
The question remains as to the mechanism by which E2 mediates its hematopoietic ERα-dependent antiaggregative action. ERαAF-10 is still able to modulate genomic signaling through its other activation function AF-2,37,38 as well as membrane-initiated/nongenomic signaling.39 Interestingly, platelets might represent a model to dissociate the acute, membrane-initiated, nongenomic effects of E2 from the genomic, long-term effects.40–44 Indeed, anucleated platelets can only respond through nongenomic means after addition of E2 in vitro. Moro et al reported an acute proaggregative effect of E2 on washed human platelets that was driven by ERβ,18 although this acute effect of E2 appears to be modest in isolated mouse platelets (M.-P.G., unpublished data, January 2011). In vivo, E2 can act on megakaryocytes through genomic and nongenomic mechanisms. Addition of E2 to megakaryocytes increases their differentiation in vitro and modulates the expression of the 2 ERs.20,45 The contrasting effects of E2 seen here in the short- versus long-term are reminiscent of the effect of E2 on macrophages. Acute E2 elicits a weak anti-inflammatory action on isolated peritoneal macrophages whereas chronic E2 has a strong proinflammatory action in vivo.46 Here we show that long-term E2 treatment modulates the platelet proteome. Very reproducibly, in 3 independent series of control and E2-treated mice, we found a decreased expression of several platelet proteins including β1 tubulin, a major constituent of microtubules which modulate platelet production and function. While this decrease, confirmed by a specific ELISA test, was significant (39%) it did not impact the number of microtubule coils in the marginal band or the size and discoid shape of resting platelets. In humans, heterozygous carriers of the Q43P β1 tubulin variant show a reduced expression of the protein associated with protection against arterial thrombosis.47 The decrease in β1 tubulin expression observed in E2-treated platelets may thus contribute to the platelet functional defects or production and the resistance to thromboembolism. However, it is likely a change in a cluster of proteins that might be responsible for the observed phenotype. In E2-treated mice, the decrease in β1 tubulin expression is accompanied by a reduced expression of other proteins including pacsin2, a protein involved in remodeling of the actin cytoskeleton, and the thioredoxin-like protein, a regulator of oxidative stress. Changes in membrane protein expression cannot be excluded because the 2D-DIGE approach is powerful for comparative cytosolic protein profiling but more limited in analyzing extreme isoelectric points or molecular weights and hydrophobic proteins. Our data strongly suggest that the change in expression of different proteins is because of ERα stimulation affecting hematopoietic progenitors or megakaryocytes, and the combined effects of these changes may in turn affect platelet responsiveness. It will be interesting to investigate the mechanisms by which E2 affects protein expression in these cells. Two transcription factors are possible targets because they are already known to regulate β1 tubulin expression in mice: the erythromegakaryocytic factor NF-E2 and GATA1.33,34 Importantly, transcriptional activity of GATA1 has been shown to be repressed by estrogens in a model of erythroid progenitor cells.48 Overall, these results strongly suggest that E2 treatment can modulate gene expression during megakaryocyte differentiation through ERα leading to modification in platelet responsiveness.
The data reported herein show promise for developing new pharmacologic strategies to prevent thrombosis. ERαAF-1 is mandatory for mediating the sexual effects of E2 in vivo21 as well as the proliferative action of E2 on breast cancer cell lines.37,49 However, in striking contrast, both the major vasculoprotective actions of E221 and, as shown here, the effects of E2 on the resistance to thromboembolism do not require AF-1. Thus, our results provide the rationale for developing selective modulators of ERα which cause minimal activation of AF-1. These would retain their beneficial cardiovascular actions but not affect sexual effects or have any proliferative action on breast cancer cells. Deciphering the molecular mechanisms elicited by E2 treatment at the level of the megakaryocyte/platelet may open new perspectives in the field of thromboprotection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the “Plateforme d'Histopatologie Expérimentale” (Dr T. Al Saati and F. Capilla), the Imaging Core Facility (Dr S. Allart), I. Fourquaux, M. J. Fouque, and the staff at the animal facility of the Institut des maladies métaboliques et cardiovasculaires of Toulouse for skillful technical assistance, C. Cenac for her technical help, and Pr L. Brouchet for helpful discussions.
This work was supported by Inserm, ANR (program Jeunes Chercheurs, Jeunes Chercheuses; no. ANR-07-JCJC-0093-01), the University of Toulouse, the Fondation de France, the Conseil Régional Midi-Pyrénées and Aquitaine, and the Société et Fédération Française de Cardiologie. M.-C.V. was supported by a grant from the Ministére de la Recherche et des Technologies (MRT). ERα+/−, ERβ−/−, and ERαAF1+/° mice were kindly provided by Prof P. Chambon and Dr A. Krust.
Authorship
Contribution: M.-C.V., M.-P.G., J.-F.A., and B.P. designed the study and wrote the manuscript; M.-C.V., M.-P.G., C.C., C.E.T., M.M., and N.S.L. performed research and collected and analyzed data; and M.-C.V., M.-P.G., P.G., F.L., P.S., M.S., J.-F.A., and B.P. critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernard Payrastre, Inserm U1048, I2MC, 1 Avenue Jean Poulhés, BP 84225, 31432 Toulouse Cedex 04, France; e-mail: bernard.payrastre@inserm.fr.
References
Author notes
J.-F.A. and B.P. contributed equally to this work.