Abstract
A mild thrombocytopenia is relatively frequent during pregnancy and has generally no consequences for either the mother or the fetus. Although representing no threat in the majority of patients, thrombocytopenia may result from a range of pathologic conditions requiring closer monitoring and possible therapy. Two clinical scenarios are particularly relevant for their prevalence and the issues relating to their management. The first is the presence of isolated thrombocytopenia and the differential diagnosis between primary immune thrombocytopenia and gestational thrombocytopenia. The second is thrombocytopenia associated with preeclampsia and its look-alikes and their distinction from thrombotic thrombocytopenic purpura and the hemolytic uremic syndrome. In this review, we describe a systematic approach to the diagnosis and treatment of these disease entities using a case presentation format. Our discussion includes the antenatal and perinatal management of both the mother and fetus.
Introduction
Thrombocytopenia, defined as a platelet count < 150 × 109/L, is second only to anemia as the most common hematologic abnormality encountered during pregnancy.1 Three large series involving together > 26 000 women suggest that its prevalence at the end of pregnancy is between 6.6% and 11.6%.2-4 However, counts < 100 × 109/L, which is the definition for thrombocytopenia adopted by an International Working Group,5 are observed in only 1% of pregnant women. The clinician's task is to determine not only the pathophysiologic nature of the thrombocytopenia, but also the risk it poses to both mother and fetus. Treatment goals change with the dynamic state of parturition and in particular during delivery, when surgical risks and the neonate's passage through the birth canal need be considered.
We discuss these problems using a case-based approach of representative clinical scenarios and examine available evidence to help guide practice. The article will focus on the clinical characteristics and management of primary immune thrombocytopenia (ITP) and its distinction from gestational thrombocytopenia, and on the thrombotic microangiopathies of pregnancy.
The majority of available reports are based on observational studies that either examine available epidemiologic evidence or report outcomes of a single therapeutic approach. Randomized trials, however, are for the most part absent. Therefore, where opinions rely on experience, we make that distinction with the goal of outlining a rational approach to diagnosis and management of pregnancy-related thrombocytopenia.
Establishing the cause of thrombocytopenia
Case 1
A 22-year-old woman from Sudan is found to have a platelet count of 82 × 109/L on routine screening at 16 weeks' gestation of her second pregnancy. Her first child was delivered at term without complications. She was told her platelet count was 62 × 109/L at that time. She has no knowledge of blood counts done at any other time. Her mother had the last of her 8 pregnancies (all uncomplicated) 2 years earlier and was found to have a low platelet count. The patient denies any history of bleeding or bruising. Her history and examination are otherwise unremarkable.
What are the most probable etiologies of her thrombocytopenia?
Causes of thrombocytopenia may be specific complications of pregnancy or have no relationship to pregnancy per se, although some of them may occur with increased frequency during gestation (Table 1). Incidental thrombocytopenia of pregnancy, usually referred to as gestational thrombocytopenia, accounts for 70%-80% of cases.2-4 It occurs in the mid-second to third trimester, and its pathogenesis is unclear. It has been speculated that it may result from various mechanisms, including hemodilution and accelerated clearance.6 No confirmatory laboratory tests are available, and the diagnosis is one of exclusion. Thrombocytopenia is typically mild to moderate, with approximately two-thirds of cases having platelet counts 130-150 × 109/L. The literature is not consistent in where a “cut-off” in platelet count is concerning; however, we consider a platelet count < 80 × 109/L as a trigger to conduct further investigations for an alternative etiology. We consider a diagnosis of gestational thrombocytopenia unlikely if the platelet count is < 50 × 109/L, with very few cases having been described with counts 40-50 × 109/L.7,8 For the thrombocytopenia to be consistent with gestational thrombocytopenia, women should have no past history of thrombocytopenia (except during a previous pregnancy), the thrombocytopenia resolved spontaneously within 1-2 months after delivery, and the fetus/newborn baby should not have had thrombocytopenia (Table 2).9
ITP is the second most common cause of an isolated low platelet count in pregnancy, accounting for ∼ 3% of women who are thrombocytopenic at delivery. Differentiating it from gestational thrombocytopenia may be problematic, if not impossible, in the absence of prepregnancy platelet counts or a previous history of ITP, as both entities are diagnoses of exclusion. As a rule of thumb, developing a platelet count < 100 × 109/L early in pregnancy, with declining platelet counts as gestation progresses, is most consistent with ITP. As usual, situations in real life are often more complicated than in guidelines. ITP may develop even during the third trimester, and platelet counts 50-80 × 109/L may also be seen in gestational thrombocytopenia. Nevertheless, knowing the exact diagnosis at that stage of pregnancy changes the management very little, as will be discussed in the indications for treatment.
In this patient, a platelet count of 82 × 109/L early in the second trimester requires further evaluation.10 A mild congenital thrombocytopenia that worsens during pregnancy cannot be excluded without prior records or testing of other family members, which should be pursued, especially because some of these disorders are associated with platelet dysfunction. Our patient reported that none of the male family members had platelet disorders. Her mother had a low platelet count during pregnancy as well, whereas a sister and cousin both had normal platelet counts and history of normal deliveries.
A rare inherited cause of thrombocytopenia is type IIB von Willebrand disease (VWD).11 Women with this condition may develop thrombocytopenia, for the first time, in pregnancy and be misdiagnosed with ITP. Platelet counts may occasionally fall to levels as low as 10-20 × 109/L at term, typically with nadir value 1-3 days before delivery, but they rapidly improve after delivery.12
What testing should be ordered when a patient presents with thrombocytopenia during pregnancy?
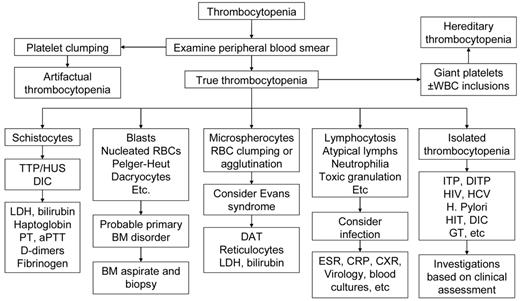
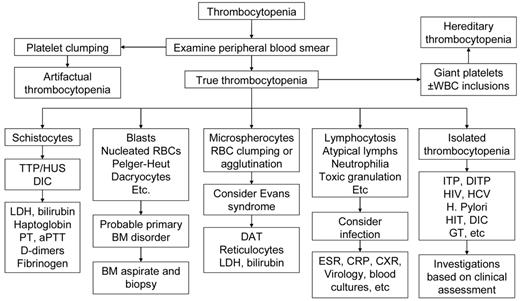
Table 3 reports the set of laboratory tests we use in our investigation of pregnant patients with thrombocytopenia. A careful review of the peripheral blood smear remains the main diagnostic procedure. Figure 1 shows our algorithm for workup of thrombocytopenia based on the observation of the peripheral blood film. Screening for coagulation abnormalities (prothrombin time, activated partial thromboplastin time, fibrinogen, D-dimers), liver function tests (bilirubin, albumin, total protein, transferases, and alkaline phosphatase), antiphospholipid antibodies and lupus anticoagulant, and serologies for systemic lupus erythematosus (SLE) are done if laboratory data, history, and physical examination suggest the thrombocytopenia may be secondary. Viral screening (HIV, hepatitis C virus [HCV], hepatitis B virus [HBV]) and Helicobacter pylori testing are recommended. Abnormalities of thyroid function are not uncommon in both ITP and pregnancy and associated with significant pregnancy-related complications and fetal risk,13 and we do this testing routinely. If the past medical history suggests frequent infections, quantitative immunoglobulin testing may be appropriate.14 If available, review of preexisting laboratory data may reveal abnormalities that preceded the pregnancy or were present only during a prior pregnancy.
Algorithm for workup of thrombocytopenia based on the observation of the peripheral blood film. The thrombocytopenia of ITP is by definition an isolated hematologic abnormality, although anemia of pregnancy or iron deficiency may also be present. Other than an occasional large form, platelets should appear normal. Consistently large and/or hypogranular platelets may suggest congenital thrombocytopenia. Uniformly small platelets are typically found in Wiskott-Aldrich syndrome. The presence of targeted red blood cells, schistocytes, macrocytosis, or spherocytes may be clues to liver disease, thrombotic microangiopathy, nutritional deficiencies, or autoimmune hemolysis. A direct antiglobulin test is necessary to rule out complicating autoimmune hemolysis (Evans syndrome). DITP indicates drug-induced thrombocytopenia; HIT, heparin-induced thrombocytopenia; and GT, gestational thrombocytopenia.
Algorithm for workup of thrombocytopenia based on the observation of the peripheral blood film. The thrombocytopenia of ITP is by definition an isolated hematologic abnormality, although anemia of pregnancy or iron deficiency may also be present. Other than an occasional large form, platelets should appear normal. Consistently large and/or hypogranular platelets may suggest congenital thrombocytopenia. Uniformly small platelets are typically found in Wiskott-Aldrich syndrome. The presence of targeted red blood cells, schistocytes, macrocytosis, or spherocytes may be clues to liver disease, thrombotic microangiopathy, nutritional deficiencies, or autoimmune hemolysis. A direct antiglobulin test is necessary to rule out complicating autoimmune hemolysis (Evans syndrome). DITP indicates drug-induced thrombocytopenia; HIT, heparin-induced thrombocytopenia; and GT, gestational thrombocytopenia.
Bone marrow examination is rarely necessary to evaluate a thrombocytopenic pregnant patient and is not required to make the diagnosis of ITP. As in the nonpregnant patient, testing for antiplatelet antibodies is of no value in the diagnosis of ITP in pregnancy, it is neither sensitive nor specific, nor is it predictive of neonatal thrombocytopenia.15,16 Type 2B VWD should be included in the differential diagnosis of thrombocytopenia during pregnancy, especially in women with a personal or family history of abnormal bleeding, or if therapy for ITP is ineffective (Table 3).
Our patient had tested negative for HIV, HBV, and HCV at initial prenatal screening. A breath test for H pylori was negative. Peripheral blood smear examination revealed a mild decrease in platelets with normal morphology and no noted abnormalities of erythrocytes or white blood cells. Thyroid testing and serum chemistries, including liver function, were unremarkable. Testing for a lupus anticoagulant, antiphospholipid antibodies, and antinuclear antibody was negative. The VWD panel was normal.
How often should the patient be monitored and what will be the indication for treatment of thrombocytopenia?
The frequency of platelet counts in pregnant women with thrombocytopenia should be based on clinical reasoning because no evidence is available. When we have a high level of confidence that a patient has gestational thrombocytopenia, we check the platelet count at the time of each routine prenatal visit. Women should also have the blood count repeated 1-3 months after delivery to assess whether spontaneous resolution of the thrombocytopenia has occurred.
If the diagnosis is ITP or uncertain, we check the platelet count every 2-4 weeks, depending on the stability of the platelet counts. If platelet counts are found to be < 80 × 109/L after week 34, we monitor them on a weekly basis.
The presence of gestational thrombocytopenia does not generally alter the management of pregnancy. However, in a few patients, the low platelet count may compromise the ability to deliver epidural anesthesia and general anesthesia represents a greater risk. For women with platelet counts of 50-80 × 109/L, in whom a diagnosis of ITP cannot be excluded, we give 10 mg of prednisone once daily starting 10 days before the anticipated date of delivery. Others may start with a higher dose and adjust downward, but there are no published randomized trials to guide management. Alternatively, some experts also advise a course of intravenous immunoglobulin (IVIg), 1 g/kg in 1 or 2 divided doses, which may be useful both diagnostically and therapeutically.17
We treat ITP in the first and second trimesters when the patient is symptomatic with bleeding, platelet counts are < 30 × 109/L, or a planned procedure requires a higher platelet count. Despite remaining relatively stable through most of the pregnancy, platelet counts may fall during the third trimester and monitoring should be more frequent. Therapy late in gestation is generally based on the risk of maternal hemorrhage at delivery (see next section).
In the case we have described, the platelet count was assessed monthly at her routine prenatal visits. Her platelet count remained 50-60 × 109/L. The frequency of hematologic monitoring was increased to weekly at 34 weeks' gestation. The fetus continued to develop normally. At 36 weeks, the platelet count fell to 43 × 109/L. The patient stated she preferred epidural anesthesia, and 10 mg of prednisone daily was begun without a response. The patient refused further therapy to increase the platelet count and was delivered without epidural of a normal boy with a cord count of 247 × 109/L. The mother's platelet count at delivery was 52 × 109/L. Two months later, her platelet count was 127 × 109/L.
Management of ITP in pregnancy
Case 2
A 36-year-old woman, gravida 2 para 2 (G2P2), with a history of ITP since age 28 asks whether she can safely become pregnant again. Her platelet count is typically 40-60 × 109/L but can fall lower after upper respiratory tract infections or at times of physical stress. She has been treated with and responded to corticosteroids and IVIg in the past. Her last pregnancy was complicated by a platelet count of 20 × 109/L in her third trimester requiring both corticosteroids and IVIg. The platelet count was 90 × 109/L at term, and she delivered a healthy neonate with a platelet count of 125 × 109/L.
What can the patient expect during a subsequent pregnancy?
A review of the clinical courses of 92 women with ITP during 119 pregnancies over an 11-year period found women with previously diagnosed ITP were less likely to require therapy for ITP than those with newly diagnosed ITP, although the frequency of bleeding complications did not differ between the 2 groups.18 Platelet counts at delivery were < 150 × 109/L in 89% of women. In most cases, thrombocytopenia was mild to moderate and the pregnancies were uneventful; however, 31% required intervention to increase the platelet count at some time during their pregnancy. Thrombocytopenia was observed in 31 of 109 (28.4%) of infants; 11 (10%) had platelet counts lower than 50 × 109/L. Two fetal deaths were recorded, one of which was caused by hemorrhage.
What treatments are safe during pregnancy?
Women with no bleeding manifestations and platelet counts > 30 × 109/L do not require any treatment until delivery is imminent. Table 4 lists currently available therapeutic options in pregnant women with ITP. First-line therapy is similar to that of nonpregnant women with newly diagnosed ITP.6,19 We usually start with oral prednisone or prednisolone at a low dose (10 mg daily). Doses are adjusted to maintain a safe platelet count, which we consider > 30 × 109/L, but hardly ever exceed 30 mg/d. Although relatively safe in pregnancy, prednisone can increase weight gain, induce hyperglycemia, exacerbate hypertension, and contribute to adverse pregnancy outcome.20 A Cochrane meta-analysis has clearly established the beneficial effects of a single course of antenatal corticosteroids (24 mg of betamethasone or dexamethasone, over 2 days) given before 34 weeks of pregnancy to accelerate fetal lung maturation when preterm delivery is imminent.21 Another meta-analysis indicates a 3.4-fold increase in risk of oral cleft among infants with first trimester exposure to steroids.22
Antenatal corticosteroids have not been found to affect the neonatal platelet count and should not be administered to the near term mother with this objective.23 Withdrawal of corticosteroids in the postpartum period must be carefully monitored and dosage tapered to avoid a rapid drop in the platelet count. If the platelet response to prednisone is suboptimal or when side effects of the drug are poorly tolerated, IVIg can be used (1 g/kg in a single or 2 divided doses), either alone or in combination with small doses of prednisone, to maintain safe platelet counts. Anti-RhD immunoglobulin is not recommended as a first-line agent because of concerns of acute hemolysis and anemia. Nevertheless, it has been used in refractory cases throughout pregnancy with successful outcomes.24 If anti-RhD (50-75 μg/kg) is administered to a patient while pregnant, the neonate should be monitored for a positive direct antiglobulin test, anemia and jaundice as the antibody may cross the placenta.
If the patient's response to first-line agents is not adequate second-line therapy may be required, although safety considerations in the pregnant patient require some change in approach. Azathioprine has been safely administered during pregnancy.25,26 Although immune impairment has been reported in some exposed infants,27 it is a reasonably safe option. High-dose methylprednisolone may also be used in combination with IVIg or azathioprine for the patient who is refractory to oral corticosteroids or IVIg alone or has a less than adequate response. Cyclosporine A has not been associated with significant toxicity to mother or fetus during pregnancy when used for inflammatory bowel disease,28 but there is no published experience on its use in ITP in pregnancy and it should be considered only when other safe second-line agents have failed.
Splenectomy may induce a remission and has been reported to be associated with few complications if performed during the second trimester,29,30 when risks of anesthesia to the fetus are minimal and uterine size will not complicate the procedure. This approach may be useful for patients who remain refractory to therapy or who experience significant toxicity with other therapies. Transplacental passage of circulating maternal antiplatelet antibodies and the risk of neonatal thrombocytopenia are not affected by splenectomy.31-33
Many of the agents frequently used in nonpregnant ITP patients may not be safe during pregnancy. Limited data are available for rituximab in pregnancy, and the use of this drug for pregnancy-associated ITP cannot be recommended because of its potential for crossing the placenta.34 Short-term therapy with danazol in combination with high-dose IVIg and corticosteroids has been used for refractory thrombocytopenia in the third trimester.35 However, danazol has been observed to cause birth defects and has been designated category X by the FDA; its use should therefore be avoided. Vinca alkaloids and cyclophosphamide are not considered safe during pregnancy. There are no published data on the use of thrombopoietin receptor agonists in pregnancy; and because their effects on the fetus are unknown, they cannot be recommended.
Thrombocytopenia secondary to SLE or the antiphospholipid antibody syndrome is generally less severe than that seen with ITP. When platelet counts are < 30 × 109/L and/or symptomatic bleeding is present, our treatment strategy is similar to that in ITP, with steroids and IVIg. In unresponsive cases, we recommend the use of azathioprine. Treatment of thrombocytopenia in these patients must always be balanced with the risk of thrombosis. Data are lacking about thrombotic risk and possible benefits of antithrombotic therapy in pregnant women with thrombocytopenia and positive antiphospholipid antibodies or lupus anticoagulant but no prior history of thrombosis. When the platelet count is > 50 × 109/L, we treat these patients with daily low-dose aspirin. If a history of prior spontaneous abortion or thrombosis is present, the patient is placed on low molecular weight heparin. In patients with ITP, we consider safe for anticoagulation a platelet count stably > 50 × 109/L.
How should delivery be managed? What is a safe platelet count for epidural anesthesia?
ITP is not an indication for cesarean delivery.6,19 Mode of delivery in a pregnant patient with ITP is based on obstetric indications, with avoidance of procedures associated with increased hemorrhagic risk to the fetus (eg, forceps, vacuum extraction, and fetal scalp electrode/samples).6 Most neonatal hemorrhages occur at 24-48 hours and are actually not related to trauma at the time of delivery.36,37 Determination of the fetal platelet count by fetal scalp vein blood draws or periumbilical blood sampling presents a potential hemorrhagic risk to the fetus and may inaccurately predict a low platelet count.38 For this reason, fetal platelet count measurement is not recommended.
Maternal anesthesia must be based on safety of the mother. The precise platelet count needed to safely perform neuraxial analgesia is unknown.39,40 American guidelines do not suggest any particular threshold but individual assessment of risks and benefits.41 Local practices may actually differ significantly. Our anesthesiologists will generally withhold spinal anesthesia for women with platelet counts < 80 × 109/L. For patients who have not required therapy during gestation but have platelet counts below this threshold, we administer short-term corticosteroids (10-20 mg) for 1-2 weeks or IVIg to raise the platelet count for the procedure. Platelet transfusion is not appropriate to prepare for spinal anesthesia; post-transfusion increments may be inadequate or short-lived and should be reserved to treat bleeding.
Uncomplicated vaginal delivery in women with ITP has been described in 1 case with platelet counts as low as 19 × 109/L,18 and in several other cases with platelet counts 20-50 × 109/L.18,42 Nevertheless, the risk of conversion to C-section is present in every labor, and we agree with current recommendations to aim for at least a level of 50 × 109/L.6 To achieve that target, various combinations of IVIG, platelet transfusions, and corticosteroids can be used. The use of platelet transfusions before delivery in pregnant women with ITP has been reported in 5%-18.9% of cases, reflecting not only different patient populations but also different practices.18,42
What is the risk to the neonate? How should he be monitored?
Platelet counts < 50 × 109/L occur in ∼ 10% of newborns of mothers with ITP, whereas platelet counts < 20 × 109/L occur in 5% of cases.18 Intracranial hemorrhage has been reported in 0%-1.5%.18,31,32 There are no indirect ways of measuring the fetal platelet count, and the correlation between maternal and fetal platelet counts is poor.4,31 The best predictor of severe thrombocytopenia at birth is its occurrence in an older sibling,2,43,44 Maternal response to treatment does not automatically protect the newborn from the development of thrombocytopenia. On the other hand, there is no robust evidence to suggest that neonates from women with ITP poorly responsive or refractory to treatment have a higher risk of severe thrombocytopenia.
A platelet count should be obtained at delivery to determine the need for immediate therapy. The count should be performed on a cord blood sample or, preferably, a peripheral blood sample. Heel-prick samples should be avoided because clots are common and may result in artifactual thrombocytopenia. If the platelet count is normal, there is no need for repeat counts, although parents should be instructed on observation for unexplained bruising or petechiae and a thorough examination performed at 1 week. In neonates with thrombocytopenia, the platelet counts is obtained daily because its nadir is frequently seen 2-5 days after birth. A spontaneous rise of the platelet count is usually seen by day 7; in some cases, however, thrombocytopenia may last weeks to months.45 We request a cranial ultrasound when the platelet count at birth is < 50 × 109/L, even in the absence of symptoms. Indeed, although intracranial bleeding may not have clinical manifestations, it requires immediate management and may affect the management of subsequent pregnancies. Until it is determined that the neonate has a safe platelet count, intramuscular injections, such as vitamin K, should be avoided. We administer platelet transfusions and IVIg (1 g/kg per day for 2 days) in all neonates with a platelet count at birth < 30 × 109/L and in those with higher platelet counts plus bleeding risk factors or active bleeding.46 We give IVIg alone to neonates who have no evidence of bleeding but have a platelet count 30-50 × 109/L. IVIg can be repeated every few weeks if needed to maintain a safe platelet count until spontaneous recovery.
Thrombotic microangiopathies of pregnancy
Preeclampsia, the HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets), and acute fatty liver of pregnancy (AFLP) have overlapping clinical and laboratory features with thrombotic microangiopathies that are not specific to pregnancy and may pose considerable diagnostic challenges (Table 5).
Case 3
A 38-year-old gravida 2 para 0 woman at 39 weeks' gestation presented to the obstetric unit after a tonic-clonic seizure witnessed by her husband. Abnormal laboratory findings included: Hb 14.7 g/dL, WBC 15.3 × 109/L, platelets 87 × 109/L, albumin 25 g/L. The urine dipstick revealed significant proteinuria (2+). Transaminases and lactic dehydrogenase (LDH) were not determined because of a hemolyzed blood sample. Coagulation results and creatinine were normal. The blood film confirmed true thrombocytopenia, with some giant platelets and a few red cell fragments.
What is the most probable etiology of her thrombocytopenia?
Preeclampsia is the second most frequent cause of thrombocytopenia developing in the late second or third trimester, accounting for 21% of cases of thrombocytopenia at the time of delivery.2 It is defined by new onset hypertension with ≥ 140 mmHg systolic or ≥ 90 mmHg diastolic blood pressure after 20 weeks' gestation together with proteinuria (≥ 0.3 g protein in a 24-hour specimen).47 Approximately 15%-25% of women with gestational hypertension will develop preeclampsia.48 In some women, the signs of preeclampsia can present as late as 4-6 weeks postpartum. There are very few data on the proportion of preeclampsia that occurs antepartum versus postpartum, but reports of the incidence of new-onset postpartum hypertension or preeclampsia range from 0.3% to 27.5%.49 The diagnosis of eclampsia is made with the onset of seizures that cannot be attributed to other causes in a woman with preeclampsia.
Thrombocytopenia may be the only initial manifestation of preeclampsia. Platelet counts < 50 × 109/L occur in < 5% of preeclamptic women.17 Intravascular hemolysis resulting from red cell fragmentation can accompany severe preeclampsia but is usually not a prominent feature. Coagulation abnormalities are unlikely if the count is > 100 × 109/L.50 Transaminases and LDH levels may be elevated, although less than seen in the HELLP syndrome.
The patient underwent an emergency cesarean delivery, and a healthy baby girl weighing 3.190 kg was delivered. A few hours after returning from the operating room, she deteriorated clinically, started passing red urine and became oliguric, and was found to have elevated liver function tests (bilirubin 45μM, alanine aminotransferase 1125 U/L, LDH 1647 U/L), raised creatinine (178μM; normal values [n.v.] < 132), low fibrinogen (1.6 g/dL), and raised D-dimers (9.75 mg/L; n.v. 0-0.3 mg/L). Her blood count showed Hb 7.7 g/dL, WBC 18.6 × 109/L, and platelets 42 × 109/L. The peripheral blood film showed thrombocytopenia with some giant platelets, several schistocytes (> 10 hpf), and polychromatic cells.
How can we explain her clinical deterioration and worsening thrombocytopenia?
The HELLP syndrome affects 10%-20% of women with severe preeclampsia, but 15%-20% of patients do not have antecedent hypertension or proteinuria.51 Criteria for the diagnosis of HELLP syndrome have been published by Sibai and include hemolysis (abnormal peripheral smear, LDH > 600 U/L, or bilirubin > 1.2 mg/dL), aspartate aminotransferase > 70 U/L, and a platelet count < 100 × 109/L.52 The Martin et al criteria are less stringent and include an LDH > 600 U/L, an aspartate aminotransferase > 70 U/L and a platelet count < 150 × 109/L.53 A partial form of the disease among women with severe preeclampsia has been described, in which only 1 or 2 of the 3 components of the syndrome are present.54 Approximately 70% of women with HELLP have evidence of the syndrome antepartum (the majority of cases are diagnosed at 28-36 weeks of gestation) and 30% develop it postpartum.55
Hemolysis has the typical features of microangiopathic hemolytic anemia, including the presence of red cell fragments (schistocytes), elevated serum bilirubin (> 1.2 mg/dL), low serum haptoglobin levels (≤ 25 mg/dL), and elevated LDH (≥ 600 IU/L) levels. Liver enzymes show variable degrees of elevation; serum transaminase levels sometimes barely exceed the upper reference limit but, in extreme cases, may be as high as 3000-4000 IU/L. Disseminated intravascular coagulation (DIC) may complicate severe cases. Thrombocytopenia, which is the earliest coagulation abnormality, defines the severity of HELLP according to the Mississippi-Triple Class System.53 The risk of serious morbidity correlates in general with increasingly severe thrombocytopenia, although severe complications, such as hepatic hemorrhage and rupture, can first appear in the patient even before the platelet count falls below 100 × 109/L.53
HELLP should be differentiated from AFLP, which is a rare, life-threatening complication of the third trimester of pregnancy, although it is not always diagnosed before delivery. Approximately 50% of patients with AFLP meet the criteria for preeclampsia, but overlap with the HELLP syndrome is also very common. In approximately 15%-20% of cases, no symptoms are present. Intrauterine death may occur. Laboratory investigations reveal several abnormalities. The blood count shows normochromic, normocytic anemia with no or mild evidence of microangiopathic hemolysis, a white blood cell count that is higher than usually seen in pregnancy and, in many but not all cases, thrombocytopenia. Thrombocytopenia is occasionally severe (platelet counts < 20 × 109/L) and is typically associated with evidence of DIC. Serum transaminase elevations (values that may exceed 1000 IU/L) are a constant feature. Bilirubin concentration, mainly conjugated, is frequently > 5 mg/dL. Other common findings include metabolic acidosis, raised creatinine (reflecting acute kidney injury often progressing to oliguric renal failure), hypoglycemia. and high ammonia. Amylase and lipase values may be elevated in the setting of concomitant pancreatitis. The diagnosis of AFLP is usually based on clinical and laboratory findings. Liver biopsy is deferred because of its inherent risk. It is rarely necessary to make the diagnosis.
What is the standard management of thrombotic microangiopathies of pregnancy?
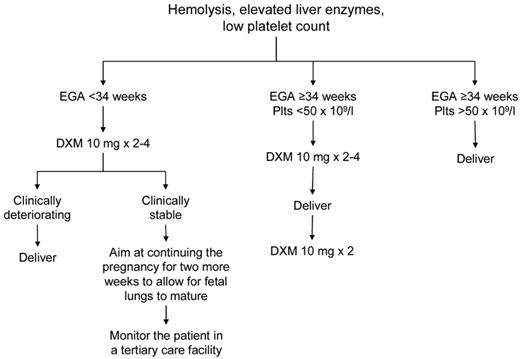
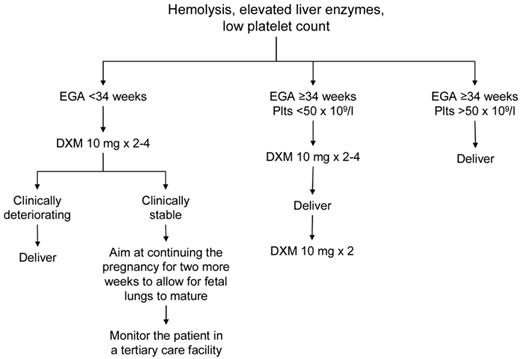
The mainstay of treatment of preeclampsia/eclampsia, HELLP syndrome, and AFLP is delivery of the fetus.56 Sudden deterioration in maternal and fetal conditions is common in pregnancies complicated by these disorders. Prompt delivery is indicated for pregnancies ≥ 34 weeks of gestation, evidence of fetal distress (based on fetal heart rate monitoring and biophysical profile), or severe maternal disease (Figure 2). For pregnancies of < 34 weeks' gestation and in which the maternal and fetal status is reassuring, glucocorticoids are recommended to accelerate fetal pulmonary maturity followed by delivery within 48 hours. Reversal of coagulopathy (eg, transfusion of fresh frozen plasma, cryoprecipitate, packed red blood cells, and platelets) may be required before delivery and/or in the immediate postnatal period. The target platelet count for safe cesarean delivery is conventionally set at > 50 × 109/L, and this is the threshold we use in our practice. We consider expectant management performed under close maternal and fetal surveillance, only in selected cases for no more than 48-72 hours and only in patients < 34 weeks of gestation. It is understood that, if maternal conditions deteriorate, cesarean delivery should be performed immediately. The presence of DIC is considered a contraindication to conservative treatment.
Suggested approach to the management of patients with HELLP syndrome. EGA indicates estimated gestational age.
Suggested approach to the management of patients with HELLP syndrome. EGA indicates estimated gestational age.
Besides delivery, the other crucial aspect in the management of preeclampsia/eclampsia, HELLP syndrome, and AFLP is prevention and treatment of seizures with magnesium sulfate. Patients may also require additional antihypertensive therapy. Most cases of preeclampsia are managed entirely by the obstetrician with minimal, if any, input from the hematologist, whose role is usually confined to confirming the presence of thrombocytopenia and ruling out other possible causes of the thrombocytopenia.
Intensive postpartum monitoring is necessary in women with HELLP because laboratory abnormalities frequently worsen 24-48 hours after delivery with peak rise in LDH and platelet nadir. In some patients, mild elevations of LDH with no evidence of ongoing hemolysis or thrombocytopenia can be observed for as long as several weeks postpartum (R.S., personal observation, June 2012). In the absence of other complications, such as severe DIC, renal dysfunction, and ascites, the platelet count should begin to rise by the fourth day postpartum and reach 100 × 109/L by the sixth day. The use of high-dose steroids in the management of HELLP syndrome remains controversial. A Cochrane meta-analysis showed that patients receiving steroids had a significantly greater improvement in platelet counts relative to those who did not, but there was no beneficial effect on maternal death or severe maternal morbidity, or perinatal/infant death.57 Dexamethasone achieves a more rapid return of the platelet count to normal than betamethasone.57 Our approach to treating patients with severe HELLP (platelets < 50 × 109/L) is to give intravenous dexamethasone 10 mg every 12 hours for 2-4 doses antepartum and 2 more doses at 10 mg intravenously every 12 hours postpartum.
AFLP usually resolves with end of pregnancy, with most patients starting to improve 2-3 days after delivery. In some cases, however, deterioration in liver function tests, renal function, neurologic status, and coagulopathy may continue for more than 1 week and require maximal supportive management in an intensive care unit. Management of severe cases should include liaison with a regional liver unit, where orthotopic transplantation may be an option.
We recommend plasma exchange if thrombocytopenia, hemolysis, or renal failure continues to worsen 48-72 hours postpartum and differentiating between preeclampsia/HELLP/AFLP and thrombotic thrombocytopenic purpura (TTP)/atypical hemolytic uremic syndrome that can follow a normal pregnancy becomes difficult, if not impossible. Several case series suggest that the use of plasma exchange treatment may improve the outcome of both severe HELLP and AFLP.58-62
Case 4
A 28-year-old primigravida at 18 weeks' gestation presented to the emergency department with a 1-week history of worsening fatigue, shortness of breath, nausea, abdominal pain, and easy bruising. On examination, the patient was pale, tachycardic, and had a few ecchymoses on her legs. Her blood count showed Hb 7.3 g/dL, WBC 12.4 × 109/L, platelets 27 × 109/L. Other abnormal laboratory tests included bilirubin 31μM and LDH 873 U/L. Renal function tests were normal. The peripheral blood film revealed a true thrombocytopenia with the presence of several large platelets, 10-20 red cell fragments per high power field, scattered spherocytes, and occasional nucleated red cells; polychromasia was increased.
What is the most probable etiology of the thrombocytopenia?
Although pathophysiologically different, TTP and HUS share overlapping clinical features and may be difficult to discern from one another, from the pregnancy-specific disorders previously described, and from some autoimmune diseases. In many reports, the clinical difference between TTP and HUS is based on the presence of acute renal failure, which is minimal or absent in the former and dominant in the latter. The incidence of TTP/HUS among all pregnancies has been estimated to be 1 in 25 000,63 significantly more frequent than in the general population.64 The time of onset of TTP/HUS in pregnancy is variable, but only a minority of cases are seen during the first trimester.
TTP is associated with a deficiency of ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), which is rarely congenital and inherited (Upshaw-Schulman syndrome) and may manifest for the first time during pregnancy. In most cases, it is an acquired disorder because of autoantibodies neutralizing ADAMTS13 activity.65 VWF-cleaving protease deficiency can also occur in DIC, HUS, preeclampsia, and HELLP.66 However, a severe deficiency (< 5% of the activity in normal plasma) appears to be specific for TTP.67
HUS is a more heterogeneous disease. The most common form of HUS (90% of cases) is caused by an infection with Shiga-toxin producing Escherichia coli (particularly types O157:H7 and O104:H4). Atypical HUS is the most common form of HUS in pregnancy and has been associated with congenital defects of the alternative pathway of the complement system.68
Early diagnosis of TTP/HUS is essential to institute treatment promptly because most fatal events occur within 24 hours from presentation in untreated subjects.69 Any patient with thrombocytopenia and microangiopathic hemolytic anemia in the absence of any obvious precipitating condition should be classified as TTP/HUS. Laboratory findings reveal evidence of microangiopathic hemolytic anemia, a negative (with rare exceptions) direct antiglobulin test, and normal coagulation tests (prothrombin time, activated partial thromboplastin time, fibrinogen and D-dimers). Renal impairment may be revealed by raised creatinine levels, with urinalysis often revealing only mild proteinuria, microscopic hematuria, and few casts.
What is the management of TTP/HUS in pregnancy?
The initial management of TTP/HUS during pregnancy does not differ from that of the nonpregnant patient. Delivery does not generally cause resolution of TTP and is not routinely indicated, although it may be required if TTP is associated with preeclampsia. Plasma exchange is the most effective therapy and should be initiated as soon as possible. Even if the diagnosis is uncertain, the potential complications of TTP-HUS exceed by far the significant risks of plasma exchange treatment.70 A virology screen on pretreatment blood samples is necessary to exclude HIV-associated TTP, and HBV and HCV before plasma exposure. Plasma exchange is initially performed daily until the platelet count is > 150 × 109/L for at least 3 days and serum LDH concentrations have returned to normal, or nearly normal, levels. We gradually taper off plasma exchange over a 2-week period. British guidelines suggest starting aspirin 75 mg/d and low molecular weight heparin at prophylactic doses (eg, dalteparin 5000 IU/d subcutaneously) once the platelet count is > 50 × 109/L66 ; these recommendations, however, are not routinely followed in other countries. If TTP develops in the first trimester, regular plasma exchange may allow continuation of pregnancy with delivery of a live infant. The frequency of the exchanges is guided by blood counts and serum lactate dehydrogenase (LDH) concentrations. Delivery is recommended for women who do not respond to plasma exchange.66 Renal failure may require supportive care with dialysis. With regard to inherited TTP, plasma infusions (10-15 mL/kg) in mothers may be sufficient, although the ideal frequency of plasma replacement during pregnancy may differ from patient to patient. Again, the frequency of plasma therapy is guided by blood counts and serum LDH concentrations. Before delivery, we recommend plasma exchange to ensure adequate levels of ADAMTS13. Close liaison with an obstetrician with expertise in maternal-fetal medicine is required. Because intrauterine fetal death may occur because of placental infarction caused by thrombosis of the decidual arterioles, serial fetal monitoring with uterine artery Dopplers should be performed. Evidence of fetal distress and intrauterine growth retardation are indications for delivery. There has been no report of transmission of TTP to the infant. Although there are reports of patients with paroxysmal nocturnal hemoglobinuria continuing eculizimab during pregnancy,71,72 there is no published experience of its use for atypical HUS in pregnancy.
The risk of relapse in subsequent pregnancies in case of inherited TTP is 100% in the absence of prophylactic plasma therapy, which should be instituted as early as possible in the first trimester.73 The risk of relapse during a subsequent pregnancy in women with acquired TTP associated with severe ADAMTS13 deficiency has been reported to be ∼ 20%.74 Our practice is to monitor women closely throughout their pregnancy and start prophylactic plasma exchange treatment if ADAMTS13 activity falls below < 10% or the blood film shows unequivocal evidence of red cell fragmentation.
In conclusion, thrombocytopenia is a relatively common occurrence in pregnancy. Diagnosis is largely dependent on timing of its onset, severity of the thrombocytopenia, and the association with other abnormalities. Isolated mild thrombocytopenia may require observation alone and present no risk to the mother or fetus. More severe thrombocytopenia or that associated with hypertension, liver abnormalities, neurologic or renal abnormalities, requires consideration of the diverse pathophysiologic mechanisms. As most treatment recommendations have been based on observational reports, questions remain as to the optimal management of the mother and safe delivery of the newborn, including safety of the TPO-receptor agonists in pregnancy and treatment of an associated prothrombotic tendency.
Authorship
Contribution: T.G., A.H.J., and R.S. performed the literature research and wrote the manuscript.
Conflict-of-interest disclosure: R.S. has served as a consultant for Amgen, GlaxoSmithKline, and Suppremol and has participated on advisory boards and/or as a speaker at medical education events supported by Amgen, GlaxoSmithKline, Nycomed, Novo, Bayer, and Baxter. The remaining authors declare no competing financial interests.
Correspondence: Roberto Stasi, Department of Haematology, St George's Hospital, Blackshaw Road, London, SW17 0QT, United Kingdom; e-mail: roberto.stasi@stgeorges.nhs.uk.
References
Author notes
T.G., A.H.J., and R.S. contributed equally to this study.