Abstract
The mouse thymus supports T-cell development, but also contains non–T-cell lineages such as dendritic cells, macrophages, and granulocytes that are necessary for T-cell repertoire selection and apoptotic thymocyte clearance. Early thymic progenitors (ETPs) are not committed to the T-cell lineage, as demonstrated by both in vitro and in vivo assays. Whether ETPs realize non–T-cell lineage potentials in vivo is not well understood and indeed is controversial. In the present study, we investigated whether ETPs are the major precursors of any non–T-lineage cells in the thymus. We analyzed the development of these populations under experimental circumstances in which ETPs are nearly absent due to either abrogated thymic settling or inhibition of early thymic development by genetic ablation of IL-7 receptorα or Hes1. Results obtained using multiple in vivo approaches indicate that the majority of thymic granulocytes derive from ETPs. These data indicate that myelolymphoid progenitors settle the thymus and thus clarify the pathways by which stem cells give rise to downstream blood cell lineages.
Key Points
Granulocytes in the adult mouse thymus share a common origin with T cells, because they derive from ETPs.
The finding that ETPs generate myeloid cells in vivo indicates that precursors settling the thymus include myelo-lymphoid progenitors.
Introduction
Since the identification of early T-lineage precursors (ETPs),1 much work has characterized the developmental potentials possessed by this population.2-13 In addition to robust T-cell developmental potential, ETPs have been shown to possess B cell, dendritic cell (DC), and natural killer (NK) cell potential and a degree of myeloid potential. As ETPs progress along the T-cell developmental pathway, they gradually lose non–T-cell potentials and generate CD4/CD8-double negative 2 (DN2) progenitors and, finally, DN3 cells that are committed to the T-cell lineage.
Single-cell assays using a stromal cell culture system have shown that the majority of individual ETPs can give rise to both T cells and myeloid cells, including granulocytes and macrophages.10,11 However, less is known about the extent to which ETPs realize this myeloid potential and other non–T-cell lineage potentials in vivo. We reported previously that approximately half of ETPs and a similar fraction of thymic granulocytes were labeled in H2-VEX V(D)J recombination reporter mice.10 These data are consistent with the notion that thymic granulocytes share a common origin with T cells. Since then, another study examining thymic myeloid cells using an IL-7 receptor (IL-7R)/Cre lineage tracing approach yielded discordant results.12 Therefore, further examination was needed to determine whether ETPs can produce granulocytes in vivo and whether ETPs are the major precursors of thymic granulocytes. An understanding of whether ETPs generate both myeloid and T-cell progeny in vivo will contribute to a more complete model of hematopoietic development.
In the present study, we examined thymic granulocyte development in experimental contexts in which ETPs are nearly absent. We reasoned that non–T-lineage cells in the thymus that are unperturbed in the absence of ETPs in mixed-BM chimeras must not predominantly derive from ETPs. Previous studies used a similar approach to investigate whether non–T-lineage cells in the thymus originate from T-cell progenitors; however, these studies did not examine thymic granulocytes.14,15 Studies using models that exclusively block intrathymic ETP development (eg, by ablating Notch signaling) may fail to detect a common origin with T-cell progenitors because progenitors continue to settle the thymus and may still generate non–T-lineage cells even when ETP development is abrogated. To address this concern, we chose to study the development of non–T-lineage cells in the thymus by eliminating T-cell progenitors before thymic entry. Specifically, we examined mixed chimeras using CCR7/CCR9 double-deficient donor BM in which T-cell progenitors display defective thymic settling and thus generate almost no ETPs. In addition, we examined thymic granulocyte development when factors necessary for early thymic development, including IL-7Rα and the Notch target Hes1, are genetically ablated. Therefore, we have undertaken multiple complementary approaches to investigate the origin of thymic granulocytes and other non–T-lineage cells in the thymus to account for possible confounding factors associated with a single approach.
Across several different in vivo experimental systems, we have consistently implicated ETPs as the major precursors of thymic granulocytes. In all of the models that we have analyzed, we have found that thymic granulocytes have distinct developmental history and developmental requirements from their extrathymic counterparts. Thymic granulocytes, like ETPs but unlike other granulocytes, show a history of RAG-1 expression and depend on CCR7/CCR9, IL-7Rα, and Hes1 for their development. These data are compatible with the notion that ETPs give rise to the bulk of thymic granulocytes in vivo. ETPs may also contribute to other non–T-lineage cells in the thymus for which they have demonstrated potential, such as macrophages, DCs, and B cells; however, these lineages derive predominantly from progenitors other than ETPs. We conclude that although ETPs possess many non–T-cell lineage potentials, they are the major precursors for only a select subset of thymic non–T-cell lineages.
Methods
Mice
Female C57BL/6 (CD45.2+) and B6.Ly5.2 (CD45.1+) mice were purchased from the National Cancer Institute and were used at 5-8 weeks of age. RAG-1/Cre mice16 were obtained from Terry Rabbitts and bred to Rosa26-YFP reporter mice. CCR9−/− mice17 were a gift from Paul Love (National Institutes of Health [NIH], Bethesda, MD). CCR7−/−CCR9−/− mice were generated by intercrossing single knockout mice. Mice with the Hes1-deficient allele were provided by Lori Raetzman (University of Illinois, Urbana-Champaign, IL) with the permission of Ryoichiro Kageyama (Kyoto University Institute for Virus Research, Kyoto, Japan). Hes1−/− mice were crossed with C57BL/6 mice. CCR7−/−, IL-7Rα−/−, and C/EBPαF/F mice18-20 were obtained from The Jackson Laboratory. All animal experiments were done according to protocols approved by the Office of Regulatory Affairs of the Perelman School of Medicine at the University of Pennsylvania (Philadelphia, PA) in accordance with guidelines set forth by the NIH.
Cell preparations, flow cytometry, and cell sorting
BM cells were obtained from mouse femurs and RBCs were lysed. Fetal liver cells were obtained from day 16.5 embryos and RBCs were lysed. Before sorting of ETPs, thymocyte cell suspensions were depleted using anti-CD4 (GK1.5), anti-CD8α (53.6-7), and magnetic beads conjugated to goat anti-rat IgG. In most experiments, the lineage Ab cocktail (Lin) used included anti-B220 (RA3-6B3) and anti-CD19 for exclusion of B cells; anti-CD11b (M1/70) and anti–Gr-1 (8C5) for exclusion of myeloid cells; anti-CD11c for exclusion of dendritic cells; anti-Ter119 for exclusion of erythroid cells; anti-NK1.1 (PK136) for exclusion of NK cells; and anti-CD3ϵ (2C11), anti-CD8α (53-6.7), anti-CD8β (53-5.8), anti-TCRβ (H57), and anti-TCRγ (GL-3) for exclusion of T-lineage cells. Additional Abs used include: anti-CD45.1(A20) and anti-CD45.2(104). Cells were sorted on a FACSAria cell sorter (BD Biosciences). Cells were analyzed on a 2-laser FACSCanto or a 4-laser LSR II flow cytometer (BD Biosciences). 4,6-diamidino-2-phenylindole was used for exclusion of dead cells. Data were analyzed using FlowJo Version 8.8.6 software (TreeStar).
IV transfers and intrathymic transfers
Female CD45.1+ host mice were irradiated with 9 Gray (Gy) and transplanted with a total of 106 BM cells. Before transplantation, BM was depleted using anti-CD4 (GK1.5), anti-CD8α (53.6-7), and magnetic beads conjugated to goat anti–rat IgG. Donor reconstitution was analyzed 8-10 weeks after transplantation. For intrathymic transfers, female CD45.1+ host mice were irradiated with 6 Gy. Intrathymic transfers were done as described previously.21 Briefly, mice were anesthetized and a thoracic incision was made to expose the thymus. Freshly sorted thymocyte progenitors were injected directly into the thymus in a 10-μL total volume.
Wright-Giemsa staining and microscopy
Sorted populations were spun onto glass slides using a Shandon cytocentrifuge. Cells were fixed in fresh methanol and stained in Wright-Giemsa reagent (Fisher Scientific) for 3 minutes, followed by Wright-Giemsa with Original Azure Blend (Harleco) for 10 minutes, then Wright-Giemsa with phosphate buffer, pH 6.8 (Fisher Scientific) for 2 minutes. Stained slides were washed with double-distilled water, allowed to dry, coverslipped, and examined under the microscope. Microscopy pictures shown in the figures are 100×. Equipment and software used include: a Leica DMRBE wide-field microscope, a QImaging MicroPublisher 5.0 MP camera, and iVision for Mac acquisition software.
PCR
For real-time PCR, messenger RNA was isolated using the RNeasy Kit (QIAGEN) and reverse transcribed with Superscript II (Invitrogen). Resultant complementary DNAs were then amplified and detected using premade TaqMan primer/probes against Hes1 and Dtx1 (Applied Biosystems). Amplification and analysis were performed on a StepOnePlus Real-Time PCR System (Applied Biosystems). Relative transcript abundance was determined by using the ΔΔCT method after normalization with GAPDH. All samples were run in triplicate. Error bars represent SEM.
Statistical analysis
Each dataset was analyzed using the Student t test on Microsoft Excel software (Version 11.5.6), with a 2-tailed distribution assuming equal sample variance.
Results
Thymic granulocytes have a developmental history of RAG-1 expression
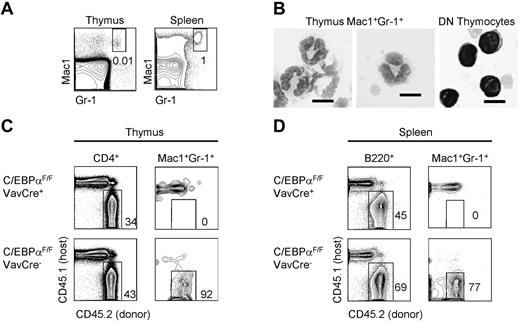
We first characterized the Mac1+Gr1+ thymic granulocyte population (Figure 1A). Thymic Mac1+Gr1+ cells had polynuclear morphology characteristic of granulocytes and distinct from lymphocytes (Figure 1B). To verify that Mac1+Gr1+ cells in the thymus were myeloid and not T-lineage cells, we made competitive BM chimeras by transplanting CD45.2+ C/EBPαF/F;VavCre+ fetal liver and CD45.1+ competitor BM into lethally irradiated CD45.1+ recipients. We verified that Mac1+Gr1+ cells in the thymus are dependent on the myeloid transcription factor C/EBPα, like granulocyte populations outside of the thymus (Figure 1C-D).22 Therefore, this Mac1+Gr1+ population is not contaminated with T lymphocytes because T-cell development is unaffected by C/EBPα deficiency (Figure 1C).22
Mac1+Gr1+ cells in the thymus are C/EBPαdependent and show polynuclear morphology. (A) Gating of Mac1+Gr1+ granulocytes in the adult mouse thymus and spleen. (B) Sorted Mac1+Gr1+ cells in the thymus and CD4/CD8 double-negative (DN) T-lymphocytes were cytospun and examined by Wright-Giemsa stain at 100×. Scale bars represent 10 μm. (C) The development of total CD4+ thymocytes and thymic granulocytes was examined in mixed chimeras of C/EBPαF/F;VavCre+ fetal liver or C/EBPαF/FVavCre− control fetal liver and congenic CD45.1+ BM. Two mice per group were examined 10 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (D) B cells and granulocyte development in the spleen were examined in mixed chimeras of C/EBPαF/F;VavCre+ fetal liver and congenic CD45.1+ BM. Two mice per group were examined 8-10 weeks after reconstitution.
Mac1+Gr1+ cells in the thymus are C/EBPαdependent and show polynuclear morphology. (A) Gating of Mac1+Gr1+ granulocytes in the adult mouse thymus and spleen. (B) Sorted Mac1+Gr1+ cells in the thymus and CD4/CD8 double-negative (DN) T-lymphocytes were cytospun and examined by Wright-Giemsa stain at 100×. Scale bars represent 10 μm. (C) The development of total CD4+ thymocytes and thymic granulocytes was examined in mixed chimeras of C/EBPαF/F;VavCre+ fetal liver or C/EBPαF/FVavCre− control fetal liver and congenic CD45.1+ BM. Two mice per group were examined 10 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (D) B cells and granulocyte development in the spleen were examined in mixed chimeras of C/EBPαF/F;VavCre+ fetal liver and congenic CD45.1+ BM. Two mice per group were examined 8-10 weeks after reconstitution.
Lymphocyte progenitors form functional antigen receptors via rearrangement of antigen receptor loci during the process of V(D)J recombination. This process requires the recombinase activating gene (RAG) enzymes RAG-1 and RAG-2, the expression of which is restricted to lymphocyte precursors. Some thymic granulocytes were previously shown to be labeled in H2-VEX V(D)J recombination reporter mice.10 Based on this finding, we wished to test directly whether ETPs can give rise to granulocytes labeled by a recombination reporter after direct intrathymic injection. For these experiments, we made use of RAG-1/Cre mice in which RAG-1 promoter elements control Cre recombinase expression.16,23 We bred these mice to mice with a floxed stop cassette upstream of the gene for yellow fluorescent protein (YFP), which has been inserted into the ubiquitously expressed Rosa26 locus. Therefore, in RAG-1/Cre × Rosa26-YFP mice, RAG-1–expressing cells and their progeny are permanently marked by YFP expression. The RAG-1/Cre mouse was superior to the H2-VEX V(D)J reporter for this experiment, which entailed analysis of rare thymic granulocyte populations because the YFP reporter provides greater fluorescence intensity and sensitivity.
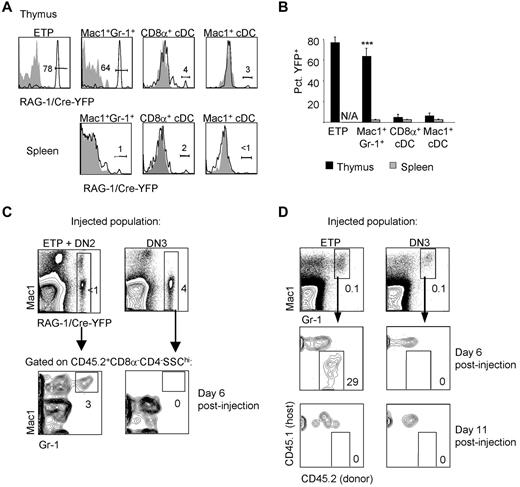
Similar to the labeling of ETPs in H2-VEX V(D)J recombination reporter mice, we found that more than half of ETPs were labeled in RAG-1/Cre × Rosa26-YFP mice (Figure 2A). We also examined whether granulocytes and other non–T-lineage cells in the thymus were labeled in these mice. Because RAG expression is restricted to lymphocyte precursors, labeling of a nonlymphoid population would suggest that this population originated from a progenitor that had expressed RAG-1 at some point during its development. We examined granulocyte and DC populations in the adult mouse thymus for a history of RAG-1 expression in RAG-1/Cre × Rosa26-YFP mice. The gating strategy used to identify these and other populations examined in this study is shown in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We found that more than half of thymic granulocytes were labeled; however, only approximately 2% of splenic granulocytes were labeled (Figure 2A-B). Thymic CD11chi conventional DCs (cDCs), including CD8α+ cDC and Mac1+ cDCs, were less than 5% labeled, which is similar to the level of labeling in splenic cDC populations (Figure 2A-B). These results are consistent with the idea that RAG-1–expressing ETPs give rise to thymic granulocytes but not thymic cDCs. Moreover, thymic granulocytes have a distinct developmental history compared with peripheral granulocytes.
ETPs generate granulocytes with a developmental history of RAG-1 expression. (A) Granulocyte and cDC subsets in the thymus and spleen were examined in RAG-1/Cre × Rosa26YFP mice for the presence of YFP labeling. Shaded histograms represent the same population in YFP− control mice. (B) The mean percentage of YFP+ cells in each population was quantified. Thymus populations are shown by black bars; splenic populations by gray bars. There is no corresponding splenic population for ETPs, which are found only in the thymus. Three mice per group were examined. Error bars represent SEM. **P < .01 for the percentage of YFP+ cells of the indicated population in the thymus compared with the analogous population in the spleen. (C) ETPs plus DN2 thymocytes or DN3 thymocytes were sorted from CD45.2+ RAG-1/Cre-Rosa26YFP mice and intrathymically injected into congenic CD45.1+ sublethally irradiated recipients (7000 ETP + DN2/recipient and 70 000 DN3/recipient). Donor contribution to YFP+ thymic Mac1+Gr1+ cells was examined 6 days after injection. (D) ETPs (10 000/recipient) and DN3 thymocytes (50 000/recipient) were sorted from CD45.2+ donor mice and intrathymically injected into sublethally irradiated CD45.1+ recipients. Mice were killed 6 or 11 days after injection and examined for donor contribution to Mac1+Gr1+ thymic granulocytes. Four mice per group were examined.
ETPs generate granulocytes with a developmental history of RAG-1 expression. (A) Granulocyte and cDC subsets in the thymus and spleen were examined in RAG-1/Cre × Rosa26YFP mice for the presence of YFP labeling. Shaded histograms represent the same population in YFP− control mice. (B) The mean percentage of YFP+ cells in each population was quantified. Thymus populations are shown by black bars; splenic populations by gray bars. There is no corresponding splenic population for ETPs, which are found only in the thymus. Three mice per group were examined. Error bars represent SEM. **P < .01 for the percentage of YFP+ cells of the indicated population in the thymus compared with the analogous population in the spleen. (C) ETPs plus DN2 thymocytes or DN3 thymocytes were sorted from CD45.2+ RAG-1/Cre-Rosa26YFP mice and intrathymically injected into congenic CD45.1+ sublethally irradiated recipients (7000 ETP + DN2/recipient and 70 000 DN3/recipient). Donor contribution to YFP+ thymic Mac1+Gr1+ cells was examined 6 days after injection. (D) ETPs (10 000/recipient) and DN3 thymocytes (50 000/recipient) were sorted from CD45.2+ donor mice and intrathymically injected into sublethally irradiated CD45.1+ recipients. Mice were killed 6 or 11 days after injection and examined for donor contribution to Mac1+Gr1+ thymic granulocytes. Four mice per group were examined.
ETPs can generate granulocytes in vivo
Labeling of ETPs and thymic granulocytes in RAG-1/Cre × Rosa26-YFP mice suggested that thymic granulocytes may derive from ETPs. To test directly whether ETPs can give rise to granulocytes with a history of RAG-1 expression, we sorted thymocyte progenitor populations from RAG-1/Cre × Rosa26-YFP mice and injected them directly into the thymus of sublethally irradiated congenic CD45.1+ recipients. We injected either pooled CD45.2+ ETPs and DN2 progenitors or DN3 progenitors and examined donor contribution to thymic granulocyte populations 6 days after injection (Figure 2C). We found that pooled ETPs and DN2 thymocytes gave rise to CD45.2+ thymic granulocytes that were YFP+, indicating a history of RAG-1 expression. As expected, control DN3 thymocytes, which are T-lineage committed, did not generate thymic granulocytes. These data show that ETPs can make granulocytes in vivo within the thymus and that these granulocytes show a history of RAG-1 expression.
We next wished to establish the kinetics with which ETP-derived thymic granulocytes can be detected after intrathymic injection. A previous study found no ETP-derived thymic granulocytes 11 or 16 days after injection12 ; however, we considered that an earlier time point may be appropriate given the short lifespan of granulocytes.24 Therefore, we intrathymically injected CD45.2+ ETPs into sublethally irradiated CD45.1+ congenic recipient mice and examined the donor contribution to thymic granulocytes 6 or 11 days after injection. Consistent with the results shown in Figure 2C, we found that ETPs gave rise to Mac1+Gr-1+ thymic granulocytes at 6 days after injection, but that these can no longer be detected 11 days after injection (Figure 2D). Again, DN3 thymocytes, which are T-lineage committed, did not generate thymic granulocytes. As expected, both ETPs and DN3 cells produced CD25+ T-lineage progeny after intrathymic injection at both time points examined (supplemental Figure 2). We conclude that ETPs have the capacity to generate granulocytes in vivo at early time points.
Impaired thymic granulocyte development in the absence of thymic settling by T-cell progenitors
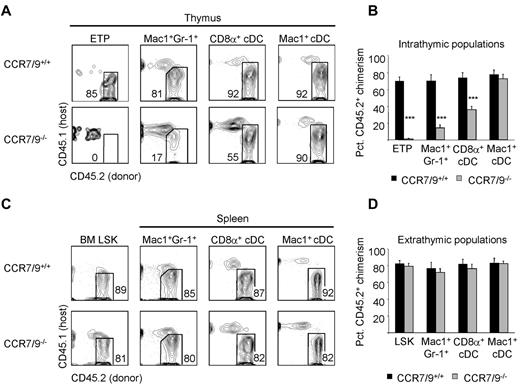
Our results with RAG-1/Cre × Rosa26-YFP mice suggested that at least a fraction of thymic granulocytes derived from ETPs. We therefore investigated whether the development of thymic granulocyte populations was dependent on the ability of T-cell progenitors to settle the thymus. Thymic settling by T-cell progenitors is impaired in mice that are doubly deficient for the chemokine receptors CCR7 and CCR9.25,26 Indeed, ETPs are almost completely absent from CCR7/CCR9 double-deficient BM in mixed BM chimeras (Figure 3A-B) despite normal engraftment of BM Lin−Sca1+Kit+ progenitors (Figure 3C-D).25 We therefore examined the ability of CCR7/CCR9 double-deficient BM to reconstitute non-T-lineage cells in the thymus of mixed BM chimeras. We reasoned that if a particular thymic lineage predominantly originates from ETPs, then the donor chimerism of this population would necessarily be reduced in the absence of CCR7 and CCR9. Alternatively, if a particular thymic lineage develops independently of both CCR7 and CCR9, then it is unlikely to develop from ETPs. Indeed, we found that the development of thymic granulocytes was diminished from CCR7/CCR9 double-deficient donor BM (Figure 3A-B), whereas splenic granulocyte chimerism was unaffected (Figure 3C-D). These results are consistent with the idea that thymic granulocytes develop from ETPs.
Thymic granulocytes are reduced in the absence of thymic settling by T-cell progenitors. (A) The development of CD45.2+ thymic ETPs, granulocytes, and cDCs in the absence of both CCR7 and CCR9 was examined in competitive BM chimeras 8-10 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (B) Shown is the mean CD45.2+ donor contribution of CCR7/CCR9+/+ (black bars) or CCR7/CCR9−/− (gray bars) BM to thymic ETPs, granulocytes, and cDCs. Eight animals per group were examined in 3 independent experiments. Error bars represent SEM. ***P < .001 for the CD45.2+ donor chimerism of the indicated population compared with BM Lin−Sca1+Kit+ (LSK) CD45.2+ donor chimerism. (C) The development of CD45.2+ BM LSK and splenic granulocytes and cDC in the absence of both CCR7 and CCR9 was examined in competitive BM chimeras 8-10 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (D) Shown is the mean CD45.2+ donor contribution by CCR7/CCR9+/+ (black bars) or CCR7/CCR9−/− (gray bars) donor BM to BM LSK, splenic granulocytes, and cDC populations. Eight animals per group were examined in 3 independent experiments. Error bars represent SEM.
Thymic granulocytes are reduced in the absence of thymic settling by T-cell progenitors. (A) The development of CD45.2+ thymic ETPs, granulocytes, and cDCs in the absence of both CCR7 and CCR9 was examined in competitive BM chimeras 8-10 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (B) Shown is the mean CD45.2+ donor contribution of CCR7/CCR9+/+ (black bars) or CCR7/CCR9−/− (gray bars) BM to thymic ETPs, granulocytes, and cDCs. Eight animals per group were examined in 3 independent experiments. Error bars represent SEM. ***P < .001 for the CD45.2+ donor chimerism of the indicated population compared with BM Lin−Sca1+Kit+ (LSK) CD45.2+ donor chimerism. (C) The development of CD45.2+ BM LSK and splenic granulocytes and cDC in the absence of both CCR7 and CCR9 was examined in competitive BM chimeras 8-10 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (D) Shown is the mean CD45.2+ donor contribution by CCR7/CCR9+/+ (black bars) or CCR7/CCR9−/− (gray bars) donor BM to BM LSK, splenic granulocytes, and cDC populations. Eight animals per group were examined in 3 independent experiments. Error bars represent SEM.
We also examined the development of cDC subsets in the thymus of CCR7/CCR9 double-deficient mixed BM chimeras. Thymic Mac1+ cDCs were unperturbed in the absence of CCR7 and CCR9; however, we consistently saw a 50% decrease in thymic CD8α+ cDC donor chimerism from CCR7/CCR9-deficient BM (Figure 3A-B). Both CD8α+ and Mac1+ cDC subsets in the spleen were unaffected by CCR7/CCR9 deficiency (Figure 3C-D). We also examined whether CCR7/CCR9 deficiency affected the development of macrophages and B-cells within the thymus. We saw a modest reduction in the development of thymic macrophages from CCR7/CCR9 double-deficient progenitors (supplemental Figure 3A-B); however, this difference did not reach statistical significance. There was a decrease in contribution of CCR7/CCR9-deficient BM to CD19hiB220hi thymic B cells (supplemental Figure 3A-B). As expected, CCR7/CCR9 deficiency did not alter macrophage or B-cell development in the spleen (supplemental Figure 3C-D). Our study cannot exclude the possibility that the absence of CCR7/CCR9 leads to reduced settling of the thymus by CD8α+ cDC, macrophages, B cells, or their precursors. In summary, these results indicate that at least 50% of thymic CD8α+ cDC, all thymic Mac1+ cDCs, and the major fraction of macrophages and B cells in the thymus develop independently of ETPs.
Thymic granulocyte development is IL-7Rα dependent
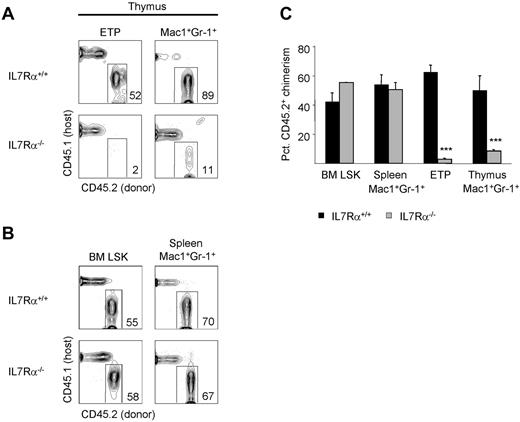
We next wished to determine whether cytokine signals critical for T-cell development are also required for the development of granulocytes in the thymus. IL-7 signaling is critical for the development of T and B lymphocytes, but is dispensable for blood granulocyte production.19,27 IL-7 signals through IL-7R, which is composed of the IL-7Rα (CD127) and the common γ-chain (CD132).28 We examined whether development of granulocytes in the thymus is dependent on IL-7R signaling; if so, this would support a model in which thymic granulocytes share a common origin with T cells. We constructed mixed BM chimeras using IL-7Rα–deficient BM and examined the development of ETPs and thymic granulocytes after 8 weeks. The IL-7Rα−/− donor chimerism of both ETPs and thymic granulocytes was reduced significantly compared with BM Lin−Sca1+Kit+ chimerism (Figure 4A-C), indicating a role for IL-7R signaling in the development of both populations. Granulocytes in the spleen were unaffected by IL-7Rα deficiency (Figure 4B). These data are consistent with the notion that most thymic granulocytes derive from T-cell precursors.
Thymic granulocyte development is IL-7Rαdependent. (A) The development of CD45.2+ ETPs and thymic granulocytes in the absence of IL-7Rα was examined in competitive BM chimeras 8 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (B) The development of CD45.2+ BM Lin−Sca1+Kit+ (LSK) and splenic granulocytes in the absence of IL-7Rα was examined in mixed BM chimeras 8 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (C) Shown is the mean percent CD45.2+ donor contribution to BM LSK, splenic granulocytes, ETPs, or thymic granulocytes by IL-7Rα+/+ (black bars) or IL-7Rα−/− (gray bars) BM. Three mice per group were examined. Error bars represent SEM. ***P < .001 for the CD45.2+ donor chimerism of the indicated population compared with BM LSK CD45.2+ donor chimerism.
Thymic granulocyte development is IL-7Rαdependent. (A) The development of CD45.2+ ETPs and thymic granulocytes in the absence of IL-7Rα was examined in competitive BM chimeras 8 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (B) The development of CD45.2+ BM Lin−Sca1+Kit+ (LSK) and splenic granulocytes in the absence of IL-7Rα was examined in mixed BM chimeras 8 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (C) Shown is the mean percent CD45.2+ donor contribution to BM LSK, splenic granulocytes, ETPs, or thymic granulocytes by IL-7Rα+/+ (black bars) or IL-7Rα−/− (gray bars) BM. Three mice per group were examined. Error bars represent SEM. ***P < .001 for the CD45.2+ donor chimerism of the indicated population compared with BM LSK CD45.2+ donor chimerism.
Thymic granulocyte development depends on the Notch target gene Hes1
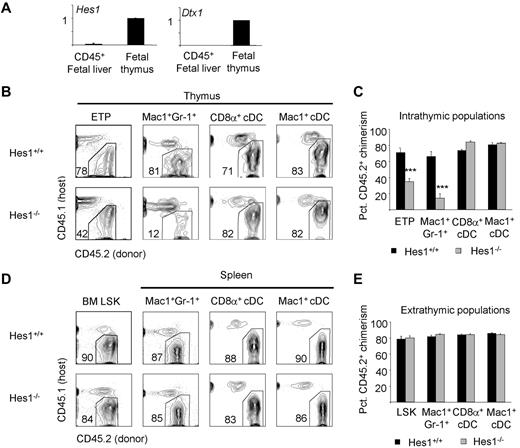
The IL-7R requirement shared by T cells and granulocytes in the thymus suggested that these cells develop from a common precursor. We next examined whether thymic granulocytes require the Notch target gene Hes1, which is required for T-cell development.29 Hes1 expression is minimal in the fetal liver and increases in the thymus downstream of Notch signaling, like the expression of another Notch target, Deltex1 (Figure 5A).30,31 Because germline Hes1 deficiency results in midgestation lethality, we constructed competitive irradiation chimeras using Hes1-deficient fetal liver progenitors. After 8-10 weeks, we analyzed the chimeric mice for donor contribution to thymic and splenic populations. We found that the contribution of Hes1-deficient cells to ETPs was decreased (Figure 5B-C), which is consistent with previous findings.32 Further, contribution of Hes1-deficient cells to thymic granulocytes was also decreased (Figure 5B-C), whereas splenic granulocytes developed independently of Hes1 (Figure 5D-E). We also examined whether Hes1 deficiency affected the development of cDCs, macrophages, and B cells within the thymus, but found no significant defects in these lineages in the absence of Hes1 (Figure 5B-C and supplemental Figure 4A-D). These data demonstrate that thymic granulocytes, like ETPs, depend on the transcription factor Hes1.
Thymic granulocyte development requires the Notch target gene Hes1. (A) Hes1 is expressed in the thymus, like another Notch target, Deltex1. Hes1 and Deltex1 expression in fetal liver are minimal. (B) The development of CD45.2+ thymic ETPs, granulocytes, and cDCs was examined in the absence of Hes1 in mixed fetal liver chimeras 8-10 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (C) Mean percent CD45.2+ donor contribution to each intrathymic lineage by Hes1+/+ (black bars) or Hes1−/− (gray bars) fetal liver. Four mice per group were examined. ***P < .001 for the CD45.2+ donor chimerism of the indicated population compared with BM Lin−Sca1+Kit+ CD45.2+ donor chimerism. (D) The development of CD45.2+ BM Lin−Sca1+Kit+, splenic granulocytes, and cDCs was examined in the absence of Hes1 in mixed fetal liver chimeras 8-10 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (E) Mean percentage CD45.2+ donor contribution to each lineage by Hes1+/+ (black bars) or Hes1−/− (gray bars) fetal liver. Four mice per group were examined. Error bars represent SEM.
Thymic granulocyte development requires the Notch target gene Hes1. (A) Hes1 is expressed in the thymus, like another Notch target, Deltex1. Hes1 and Deltex1 expression in fetal liver are minimal. (B) The development of CD45.2+ thymic ETPs, granulocytes, and cDCs was examined in the absence of Hes1 in mixed fetal liver chimeras 8-10 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (C) Mean percent CD45.2+ donor contribution to each intrathymic lineage by Hes1+/+ (black bars) or Hes1−/− (gray bars) fetal liver. Four mice per group were examined. ***P < .001 for the CD45.2+ donor chimerism of the indicated population compared with BM Lin−Sca1+Kit+ CD45.2+ donor chimerism. (D) The development of CD45.2+ BM Lin−Sca1+Kit+, splenic granulocytes, and cDCs was examined in the absence of Hes1 in mixed fetal liver chimeras 8-10 weeks after reconstitution of lethally irradiated CD45.1+ hosts. (E) Mean percentage CD45.2+ donor contribution to each lineage by Hes1+/+ (black bars) or Hes1−/− (gray bars) fetal liver. Four mice per group were examined. Error bars represent SEM.
Discussion
The earliest progenitors of T cells in the mouse thymus retain a variety of alternative or non-T-lineage potentials; however, it has remained controversial whether these potentials are realized within the thymus in vivo. Using multiple in vivo models, we found in the present study that thymic granulocytes have a distinct origin from peripheral granulocytes. Furthermore, our study implicates ETPs as the major precursors of thymic granulocytes. Thymic granulocytes differ from their peripheral counterparts in several ways that suggest a close developmental relationship with T-cell progenitors: (1) they are labeled by a history of RAG-1 expression, (2) they are diminished in the absence of chemokine receptors necessary for thymic settling by T-cell progenitors, and (3) they rely on IL-7Rα and the Notch target gene Hes1 for their development. These properties together provide strong evidence that thymic granulocytes and T cells derive from the same precursors.
A recent study using an IL-7R/Cre lineage tracing approach found that less than 20% of Mac1+Gr1+ thymic granulocytes were labeled with a developmental history of IL-7R expression and the investigators concluded that T cells and myeloid cells in the thymus have separate origins.12 However, we found that thymic granulocytes depend on IL-7Rα for their development. The simplest explanation for this apparent discrepancy is that lineage tracing approaches require a threshold level of Cre recombinase expression, whereas cytokine receptors can be active at levels below this threshold.33 Therefore, cellular responsiveness to cytokines is a more sensitive readout than reporter labeling.34 Thus, although thymic granulocyte precursors do not reach the threshold level of Cre expression needed to confer labeling in IL-7R/Cre reporter mice, they were clearly revealed to require IL-7Rα in our studies. Our results argue against the conclusions reached using the IL-7R/Cre lineage tracing approach and reveal that most thymic granulocytes share a common origin with T cells.
Because Notch signaling inhibits non–T-cell fates,10,35-38 it has been proposed that ETPs may give rise to non–T-lineage cells instead of becoming T cells when they “escape” strong Notch signals. Indeed, the Notch target gene Hes1 is specifically required for T-cell development and dispensable for peripheral myeloid development.29,32 Moreover, Notch and Hes1 can inhibit myeloid development.10,35,36 However, we found that thymic granulocytes were diminished in the absence of Hes1. These data show that thymic granulocytes develop from a progenitor that requires Hes1, like T-cell precursors but unlike extrathymic granulocytes. Interestingly, elements of the T-cell developmental program, such as Gfi1, are both expressed by ETPs39 and are involved in neutrophil fate specification.40 It is possible that components of the early T-cell developmental program allow and perhaps even potentiate the granulocyte fate from some uncommitted progenitors.
The results of the present study indicate that cells of the same lineage can arise from distinct progenitors in different sites. Similarly, DCs can derive from either myeloid or lymphoid progenitors.41 We verified that thymic Mac1+Gr1+ cells have polynuclear morphology and depend on the transcription factor C/EBPα for their development, thus confirming their identity as myeloid cells. Although the function of thymic granulocytes is notknown precisely, it is possible that these cells act as scavengers of apoptotic thymocytes. Because the majority of developing thymocytes will fail positive or negative selection, scavenger cells such as macrophages play an important role in thymopoiesis.42,43 A recent study showed that mice deficient for neutrophil migration were impaired in their ability to clear apoptotic cells after irradiation.44 Therefore, it is possible that granulocytes function as scavengers of apoptotic thymocytes in the steady state.
Granulocytes are unique among non–T-lineage cells in the thymus in that their major precursor appears to be ETPs. Our data suggest that there are no other efficient sources of thymic granulocytes apart from ETPs. The frequencies of donor-derived thymic macrophages and B cells from CCR7/CCR9 double-deficient progenitors were reduced compared with wild-type controls (25% and 37%, respectively) and for B cells this achieved statistical significance (supplemental Figure 3). We cannot exclude the possibility that the absence of CCR7 and/or CCR9 leads to reduced settling of the thymus by macrophages, B cells, or their precursors. However, our results are consistent with other work that examined the development of thymic macrophages and B cells and concluded that despite the demonstrated macrophage and B-cell potential of ETPs,2,4-7,10,11,13 the major fraction of these thymic lineages develop independently of ETPs.11,15 Therefore, ETPs may contribute to lineages in the thymus other than granulocytes, but there are likely to be more efficient or abundant precursors for thymic macrophages and B cells than ETPs. It is important to note that our conclusions apply only to adult mice. The frequency of ETPs with B-lineage potential is increased in neonatal mice,13,45 and ETPs may be a more significant source of thymic B cells in early life.
ETPs have been shown to have DC potential in vitro and in vivo on intrathymic injection.3,46 Specifically, CD8α+ thymic cDCs have been proposed to originate from ETPs.47,48 We found that thymic cDC subsets are unlabeled in RAG-1/Cre reporter mice, which is consistent with previous reports that this population lacks TCR rearrangements.15 Although thymic cDC development can be uncoupled from T-cell development in the context of Notch1 deficiency,14,15 these studies cannot exclude the possibility that thymic cDCs develop from thymus-settling progenitors upstream of ETPs. In the absence of Notch, progenitors continue to arrive at the thymus and fail to become ETPs, but may develop into other lineages. Therefore, we investigated whether thymic cDC development would be perturbed by deficiency for CCR7 and CCR9, which results in near complete ablation of ETPs in competitive chimeras. We found that approximately half of CD8α+ thymic cDCs derive completely independently of ETPs in CCR7/CCR9-deficient mixed BM chimeras. We cannot exclude the possibility that the absence of CCR7 and/or CCR9 leads to reduced settling of the thymus by DC or DC progenitors. In addition, CD8α+ thymic cDCs are not perturbed in the context of Hes1 deficiency despite substantial reductions in ETPs. Consistently, a recent study49 proposed that thymic CD8α+ cDCs derive from DN1c thymocytes, which are not considered canonical T-cell progenitors.50 These data indicate that most or all thymic cDCs, including the CD8α+ subset, derive independently of ETPs.
In summary, this study establishes that T cells and granulocytes in the adult mouse thymus derive from a common progenitor. ETPs may generate other non–T-lineage cells in the thymus; however, ETPs are not the major precursors of these cells (supplemental Figure 5). These data, together with other recent work,13 support the notion that some thymus-settling progenitors are myelolymphoid progenitors. Further, the myeloid potential of uncommitted T-cell progenitors is realized in vivo in the adult mouse thymus. Future studies are needed to clarify the mechanisms that control the lineage fate decisions of ETPs and their descendants.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank R. Kageyama for permission to use Hes1−/− mice; P. Love for CCR9−/− mice; T. Rabbitts for RAG-1/Cre mice; and David Allman, Dan Zlotoff, and Will Bailis for critical comments on the manuscript.
This work was supported by National Institutes of Health (grants R01-AI059621 and R01-HL110741 to A.B., grants T32 GM-07229 and T32 CA-09140 to M.E.D., and grant 1-F32-AI-080091-01A1 to J.J.B).
National Institutes of Health
Authorship
Contribution: M.E.D., J.J.B., and A.B. designed and performed the experiments and analyzed the data and M.E.D. and A.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Avinash Bhandoola, 266 John Morgan Bldg, 3620 Hamilton Walk, Philadelphia, PA 19104; e-mail: bhandooa@mail.med.upenn.edu.