Abstract
Immunoglobulin class switching from IgM to IgG in response to peptides is generally T cell–dependent and vaccination in T cell–deficient individuals is inefficient. We show that a vaccine consisting of a dense array of peptides on liposomes induced peptide-specific IgG responses totally independent of T-cell help. Independency was confirmed in mice lacking T cells and in mice deficient for MHC class II, CD40L, and CD28. The IgG titers were high, long-lived, and comparable with titers obtained in wild-type animals, and the antibody response was associated with germinal center formation, expression of activation-induced cytidine deaminase, and affinity maturation. The T cell–independent (TI) IgG response was strictly dependent on ligation of TLR4 receptors on B cells, and concomitant TLR4 and cognate B-cell receptor stimulation was required on a single-cell level. Surprisingly, the IgG class switch was mediated by TIR-domain-containing adapter inducing interferon-β (TRIF), but not by MyD88. This study demonstrates that peptides can induce TI isotype switching when antigen and TLR ligand are assembled and appropriately presented directly to B lymphocytes. A TI vaccine could enable efficient prophylactic and therapeutic vaccination of patients with T-cell deficiencies and find application in diseases where induction of T-cell responses contraindicates vaccination, for example, in Alzheimer disease.
Key Points
Peptide assemblies on MPLA-containing liposomes induce IgG responses independent of T cells, CD40L, CD28, CD14, and MyD88.
Direct stimulation of B cells through TLR4 and TRIF is required for T cell–independent IgG responses.
Introduction
Most peptide and protein antigens require T-cell help for B-cell activation and antibody production,1 while polysaccharides and lipopolysaccharide (LPS) can stimulate antibody responses without T help.2 In both T cell–independent (TI) and –dependent (TD) antibody responses, cognate antigens bind their B-cell receptor (BCR). TD antigens are internalized, processed, and presented in the context of MHC class II molecules to antigen-specific T-helper cells.3 The activated T cell then facilitates B-cell responses and immunoglobulin isotype switching via costimulatory molecules, adhesive antigens, and cytokines. TI antigens typically stimulate transient IgM antibody production with little or no IgG, IgA, or IgE production. TI type 1 (TI-1) antigens are polyclonal B-cell activators such as LPS whereas TI type 2 (TI-2) antigens consist of repetitive biochemical structures, such as polysaccharides or glycoproteins.4
Liposomes are submicron vesicles of phospholipids and allow integration of compounds within and on the lipid bilayer. Antigens can be packed on the surface to resemble TI-2 antigens; in nature, repetitive arrangement of a single protein or peptide on cells or microorganisms does not typically occur. In the present study, a peptide was palmitoylated and mixed with phospholipids and monophosphoryl lipid A (MPLA) to allow formation of liposomes with densely arranged peptides on the outer surface. The palmitoylated human β-amyloid (Aβ, aa1-15) peptide adopts an aggregated β-sheet conformation on liposomes.5 While previous reports have suggested that nonreplicating protein vaccines do not enable TI isotype switching,6,7 the present study in mice demonstrates that switch is feasible with a correct structural assembly of antigen and adjuvant. This result could pave the way for vaccination of patients with T-cell deficiencies or elderly, or when T-cell involvement is associated with immunopathology or autoimmunity, for example, encephalitis in Alzheimer disease.
Methods
Mice
Mice deficient for CD14 (B6.129S-Cd14tm1Frm/J), CD28 (B6.129S2-Cd28tm1Mak/J), CD40L (B6.129S2-Cd40lgtm1Imx/J), TCR αβ and γδ (B6.129P2-Tcrbtm1Mom Tcrdtm1Mom/J), MHC class II (B6.129S2-Ciitatm1Ccum/J), MyD88 (B6.129P2(SJL)-Myd88tm1Defr/J), TIR-domain-containing adapter inducing interferon-β (TRIF; C57BL/6J-Ticam1Lps2/J), μMT (B6.129S2-Ighmtm1Cgn/J) as well as nude mice (B6.Cg-Foxn1nu/J) were purchased from The Jackson Laboratory. Wild-type (WT) C57BL/6 mice were purchased from Charles River. All treatments were approved by the Local Committee for Animal Use and were carried out in accordance to state and federal regulations.
Preparation of liposomes
Liposomes containing the palmitoylated human Aβ antigen aa1-15 [H-K(Palm)-K-(Palm)-DAEFRHDSGYEVHHQ-K(Palm)-K(Palm)-OH], short PalmAβ1-15, (Bachem) were prepared from dimyristoyl phosphatidyl choline (Lipoid), dimyristoyl phosphatidyl glycerol (Lipoid), cholesterol (Solvay), and MPLA (Avanti Polar Lipids) at molar ratios 9:1:7:0.06.5,8 The liposomes were sterilized by filtration and endotoxin free. Each dose (200 μL) contained 78 μg of PalmAβ1-15 and 12 μg of MPLA.
Preparation of OVA-Alum vaccines
Ovalbumin (OVA; Sigma-Aldrich) in PBS (1 mg/mL), was mixed with aluminum hydroxide (Alum; Alhydrogel, Brenntag Biosector) to obtain a final concentration of 5 mg/mL Alum. The OVA/Alum solution was mixed with CpG (CpG ODN 1668, phosphorothioate-modified as indicated by asterisks in the 5′-3′ sequence: T*CC ATG ACG TTC CTG A*C*G *T*T; Microsynth). Each vaccine dose of 200 μL contained 100 μg of OVA, 1000 μg of Alum, and 60 μg of CpG.
Immunizations
Mice were immunized subcutaneously (s.c.) with 200 μL of liposomes at various intervals. Blood serum samples prepared before and at various time points after immunization were frozen at −20°C for later antibody analysis by ELISA. For analysis of T-cell responses, mice were immunized s.c. with Aβ1-15 liposomes or with OVA, Alum, and CpG on days 0 and 10. On day 15, spleens were harvested for cell-proliferation assay and ELISPOT analysis.
Germinal center analysis by immunohistochemistry and flow cytometry
Wild-type, athymic and CD28−/−, CD40L−/−, or TCR−/− mice were immunized with liposomes containing Aβ1-15 and MPLA as described in “Immunizations,” and spleens were harvested for the analysis of germinal centers (GCs) and activation-induced cytidine deaminase (AID) expression. Half of the spleen was snap-frozen for immunohistology, while half the spleen was freshly homogenized for analysis by flow cytometry. The frozen sections were stained with peanut agglutinin PNA, anti–GL-7, or anti-AID (eBioscience). GL-7– and B220-positive B cells in erythrocyte-freed splenocytes were analyzed by flow cytometry (eBioscience). Acquisition was done with FACSCanto (BD Biosciences) and analysis with FlowJo 8.5.2 (TreeStar).
Quantification of OVA- and Aβ-specific cytokine producing T cells by ELISPOT
Cytokine production of OVA- or Aβ-specific splenocytes was assessed by ELISPOT according to the manufacturers' instructions (Mabtech) after incubation of cells with 2μM OVA, 2μM Aβ1-42, or 5 μg/mL Concanavalin A (GE Healthcare) at 37°C and 5% CO2 for 48 hours. The plates were then washed and incubated with biotinylated anti–mouse IFN-γ or IL-4 monoclonal antibodies and developed using streptavidin-alkaline phosphatase (AP), substrate BCIP-NBT.
Measurement of Aβ-specific and OVA-specific antibody responses
Aβ1-42–specific and OVA-specific IgG and IgM antibodies were determined by ELISA. Plates were coated with 10 μg/mL Aβ1-42 (Bachem) or 30 μg/mL OVA overnight at 4°C and blocked with 1% BSA. Serial dilutions of serum (1/100 to 1/12 600) were analyzed with AP-conjugated anti-mouse IgG total antibody (Jackson ImmunoResearch Laboratories), HRP-conjugated IgM antibody (BD Bioscience/BD Pharmingen), or subclass-specific antibodies (IgG1-AP, IgG2a/c-biotin, IgG3-biotin; BD Pharmingen) or IgG2b-AP (Zymed Laboratories). For IgG2a/c,9 and IgG3 Streptavidin-HRP was used. The ELISAs were developed with AP (pNPP) or HRP (ABTS) substrates. The results for IgG are expressed with reference to the antibody 6E10 (Covance) and as optical density (OD) at a nonsaturated plasma dilution for IgM, IgG1, IgG2a/c, IgG2b, and IgG3.
CDR sequencing
The antibody variable regions of 3 antibodies obtained from mice immunized with Aβ liposomes were sequenced and compared with germline murine immunoglobulin sequences in the NCBI database. Differences exactly at the VDJ or V to J joining points were not considered as sequence changes attributable to affinity maturation.
Biacore assay
Surface plasmon resonance (SPR) binding measurements were carried out on a Biacore T100 instrument (GE Healthcare) at 25°C using PBS as running buffer. Acetylated human Aβ antigen aa1-15 [H-K(Ac)-K-(Ac)-DAEFRHDSGYEVHHQ-K(Ac)-K(Ac)-OH], short Acetyl1-15, (PolyPeptides) was solubilized in acetate buffer pH 4.0 at 4 mg/mL and immobilization on a Serie S sensor chip CM5 (GE Healthcare) using amine coupling, giving a final immobilization level of 1239 RUs. Sera from wild-type and nude mice immunized with PBS or Aβ-peptide liposomes sampled at day 7 and day 56 were assessed. Four serial dilutions between 50- and 400-fold were assayed using running buffer at a flow rate of 50 μL/min for 120 seconds. After injection was finished, the surfaces were washed immediately with running buffer for 240 seconds. Surface regeneration was performed by injecting one time 2.5 μL of 10mM Glycine-HCl pH 1.7.
Binding to the negative control surface (underivatized flow cell 1) was subtracted from each sample curve and maximal response unit was measured at 120 seconds postinjection. Normalized dissociation was calculated as a ratio of late to early binding responses over a dissociation phase of 360 seconds.10 For each serum, the normalized dissociation obtained at days 7 and 56 were compared from dilutions giving binding curves with similar maximal response unit.
ELISA for avidity measurements
Avidity measurements of serum antibodies were performed as described.11 The ELISA was optimized so that the amount of antibody binding to the coated peptide represented < 10% of antibodies in the liquid phase. Each serum was adjusted to an optimal concentration (OD of 3.0) and was incubated with different concentrations of Aβ1-42 for 2 hours at 37°C. The serum was transferred to the coated plates and the ELISA performed as described in “Measurement of OVA- and Aβ-specific antibody responses.” The IC50 values were calculated by “log (inhibitor) vs response” fitting using GraphPad Prism software.
Cryo-TEM measurements
Liposomes (4-5 μL) were applied to an EM grid with a holey carbon film. The grid was blotted and frozen, and the image acquisition was made at −170°C in a Philips CM12 electron microscopy (FEI Electron Optics) equipped with a Gatan 626 cryospecimen holder and a magnification of ×22 000. Digital images were recorded with a Gatan MultiScan charge-coupled device (CCD) camera and processed with Gatan Digital Micrograph software.
Adoptive transfer
Single-cell suspensions of splenocytes were prepared from TLR4-deficient, TRIF-deficient, or WT mice after red blood cell lysis. The cells were washed and resuspended in PBS, and 100 million cells were injected intravenously into each μMT mouse, which were immunized as described in “Immunizations” with Aβ1-15 and MPLA liposomes 18 hours later.
Statistical analysis
All statistical analyses were performed using Prism Version 5 (GraphPad Software). Data were analyzed by 1-way or 2-way ANOVA followed by Tukey posthoc analysis for multiple comparisons or by 2-sided Kruskal-Wallis nonparametric tests (when n < 10). The significance level was set at 0.05.
Results
Aβ-peptide liposomes stimulate rapid Aβ-specific IgG antibody responses with no evidence of T-cell activation
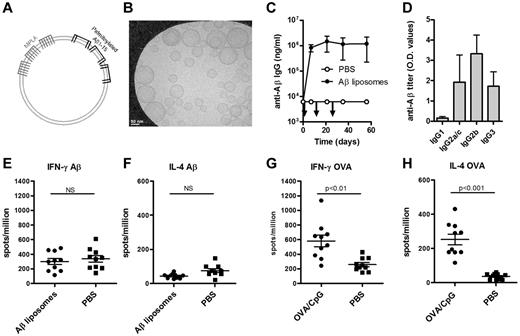
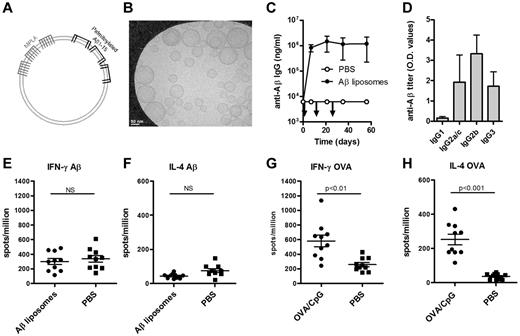
The Aβ1-15 peptide is anchored in the unilaminar bilayer through 4 palmitoyl chains, and the adjuvant MPLA is integrated into the lipid bilayer through 6 fatty acid chains (Figure 1A-B). Immunization of wild-type (WT) C57BL/6 mice with Aβ1-15 liposomes triggered strong anti-Aβ IgG responses (> 100 000 ng/mL) within 7 days, and repeated injections on days 14 and 28 did not boost the titers (Figure 1C). IgG2b was the dominant subclass, but high titers of IgG3 and IgG2a/c were also measured (Figure 1D). Both the rapid appearance of IgG antibodies and the stimulation of IgG3 production suggested a TI antibody production.12
Immunization with Aβ-peptide liposomes induces a rapid anti-Aβ IgG antibody response but no T-cell activation. (A) Schematic representation of Aβ-peptide liposomes. Aβ1-15 is attached to liposomes by palmitoyl chains coupled to the side chain of 4 lysines. The adjuvant MPLA integrates into the liposomes through fatty acid chains. (B) Cryoelectron microscopy of Aβ-peptide liposomes. The liposomes consist of unilamellar bilayers. Scale bar shows 50 nm. (C) Anti-Aβ IgG antibodies in the plasma of C57BL/6 mice immunized with Aβ-peptide liposomes or PBS. Mice were injected s.c. at days 0, 14, and 28 (as indicated by arrows) and bled before and 7, 21, 35, and 56 days after the first immunization. Results are expressed as mean ± SD (n = 9 per group). The data are representative of more than 10 independent experiments. (D) Anti-Aβ IgG subclasses in the plasma of C57BL/6 mice receiving Aβ-peptide liposomes or PBS at day 35. Results are expressed as OD values at a dilution of 1/400 and shows mean + SD (n = 9 per group). The data are representative of more than 5 independent experiments. (E-H) IFN-γ and IL-4 production of splenocytes from immunized mice restimulated with (E-F) Aβ1-42 or (G-H) OVA in vitro. C57BL/6 mice were injected s.c. at days 0 and 10 with Aβ-peptide liposomes or OVA/CpG/Alum and analyzed for cytokine production 15 days after the first immunization. Results are expressed for 10 individual mice per group with the mean indicated by a line. NS indicates not significant.
Immunization with Aβ-peptide liposomes induces a rapid anti-Aβ IgG antibody response but no T-cell activation. (A) Schematic representation of Aβ-peptide liposomes. Aβ1-15 is attached to liposomes by palmitoyl chains coupled to the side chain of 4 lysines. The adjuvant MPLA integrates into the liposomes through fatty acid chains. (B) Cryoelectron microscopy of Aβ-peptide liposomes. The liposomes consist of unilamellar bilayers. Scale bar shows 50 nm. (C) Anti-Aβ IgG antibodies in the plasma of C57BL/6 mice immunized with Aβ-peptide liposomes or PBS. Mice were injected s.c. at days 0, 14, and 28 (as indicated by arrows) and bled before and 7, 21, 35, and 56 days after the first immunization. Results are expressed as mean ± SD (n = 9 per group). The data are representative of more than 10 independent experiments. (D) Anti-Aβ IgG subclasses in the plasma of C57BL/6 mice receiving Aβ-peptide liposomes or PBS at day 35. Results are expressed as OD values at a dilution of 1/400 and shows mean + SD (n = 9 per group). The data are representative of more than 5 independent experiments. (E-H) IFN-γ and IL-4 production of splenocytes from immunized mice restimulated with (E-F) Aβ1-42 or (G-H) OVA in vitro. C57BL/6 mice were injected s.c. at days 0 and 10 with Aβ-peptide liposomes or OVA/CpG/Alum and analyzed for cytokine production 15 days after the first immunization. Results are expressed for 10 individual mice per group with the mean indicated by a line. NS indicates not significant.
Full-length Aβ peptide (Aβ1-42) contains T-cell epitopes, while truncated Aβ1-15 used here lacks known T-cell epitopes.13 Consequently, no detectable antigen-specific secretion of IFN-γ (Figure 1E) or IL-4 (Figure 1F) was observed in splenocytes restimulated in vitro with Aβ1-42. In contrast, significant T-cell responses were observed in mice immunized with the TD antigen OVA (Figure 1G-H). Antigen-specific proliferation was also observed in cells from OVA but not from Aβ1-15–immunized mice (data not shown).
Antibody responses induced by Aβ-peptide liposomes are T-cell independent
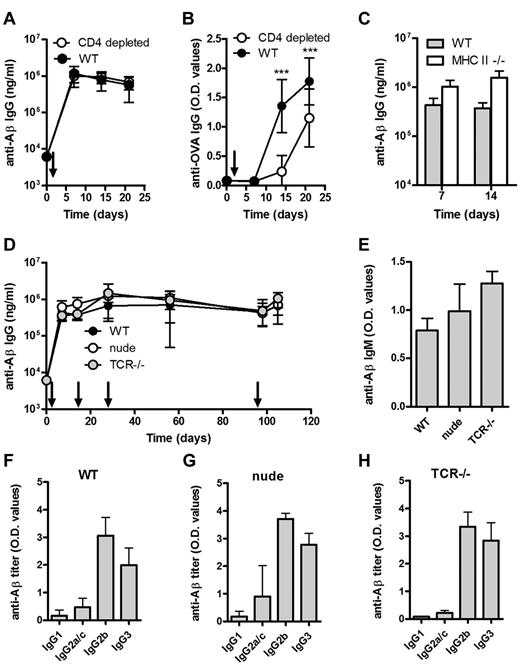
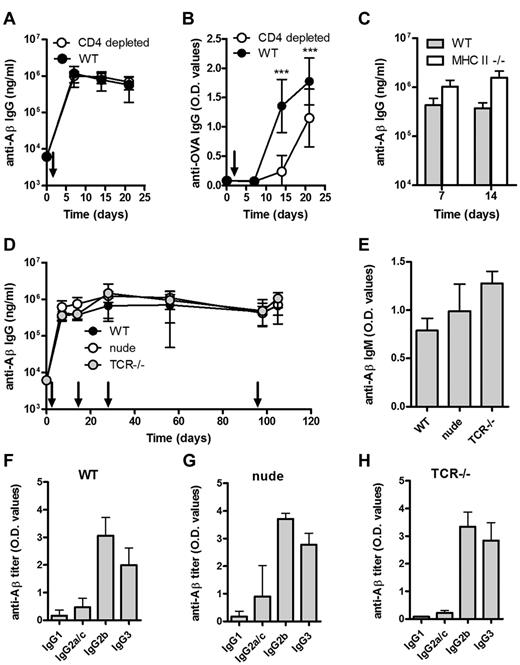
WT mice depleted of CD4+ T cells and immunized with Aβ1-15 liposomes produced a robust anti-Aβ IgG response, which did not differ from nondepleted mice (Figure 2A). For comparison, CD4 T-cell depletion reduced OVA-specific IgG responses in mice immunized with OVA (Figure 2B). The CD4 T-cell depletion (> 98%) was confirmed by flow cytometric analysis (data not shown), but to rule out a potential contribution by residual CD4+ T cells, the same experiment was performed in MHC II–deficient mice, which lack mature CD4 T cells. Again, Aβ1-15 liposomes induced anti-Aβ IgG responses comparable with those observed in WT mice (Figure 2C).
Aβ-peptide liposomes induces a rapid, robust, and persistent antibody response independent of CD4+ T cells, α/β T cells, and γ/δ T cells. (A) Anti-Aβ or (B) anti-OVA IgG antibodies in the plasma of WT mice or CD4-depleted WT mice immunized with Aβ-peptide liposomes or OVA/Alum, respectively; depletion was done by weekly i.p. injections of 100 μg (optimized in house, data not shown) anti-CD4 antibody (clone YTS191.1; AbD Serotec) starting 3 days before immunization, and verified by flow cytometry on blood sampled on days 0, 7, 14, and 21. Results are expressed as mean ± SD (n = 8 per group). Arrows indicate time of immunization (d0); ***P < .001. (C) Anti-Aβ IgG antibodies in the plasma of MHC II−/− and WT mice receiving Aβ-peptide liposomes at day 0 and 7. Results are expressed as mean + SD (n = 6 per group). (D) Anti-Aβ IgG antibodies in the sera of WT, nude, and TCR−/− mice receiving Aβ-peptide liposomes (as indicated by arrows). Results are expressed as mean ± SD (n = 6 per group). (E) Analysis of anti-Aβ IgM in the sera of WT, nude, and TCR−/− mice from day 7. Results are expressed as mean OD values at a dilution of 1/100 + SD (n = 6 per group). (F-H) Analysis of anti-Aβ IgG subclasses in the sera of WT, nude, and TCR−/− mice receiving Aβ-peptide liposomes. Analysis of IgG subclasses were done on sera prepared from blood on day 28. Results are expressed as mean + SD OD values at a dilution of 1/400 (n = 6 per group).
Aβ-peptide liposomes induces a rapid, robust, and persistent antibody response independent of CD4+ T cells, α/β T cells, and γ/δ T cells. (A) Anti-Aβ or (B) anti-OVA IgG antibodies in the plasma of WT mice or CD4-depleted WT mice immunized with Aβ-peptide liposomes or OVA/Alum, respectively; depletion was done by weekly i.p. injections of 100 μg (optimized in house, data not shown) anti-CD4 antibody (clone YTS191.1; AbD Serotec) starting 3 days before immunization, and verified by flow cytometry on blood sampled on days 0, 7, 14, and 21. Results are expressed as mean ± SD (n = 8 per group). Arrows indicate time of immunization (d0); ***P < .001. (C) Anti-Aβ IgG antibodies in the plasma of MHC II−/− and WT mice receiving Aβ-peptide liposomes at day 0 and 7. Results are expressed as mean + SD (n = 6 per group). (D) Anti-Aβ IgG antibodies in the sera of WT, nude, and TCR−/− mice receiving Aβ-peptide liposomes (as indicated by arrows). Results are expressed as mean ± SD (n = 6 per group). (E) Analysis of anti-Aβ IgM in the sera of WT, nude, and TCR−/− mice from day 7. Results are expressed as mean OD values at a dilution of 1/100 + SD (n = 6 per group). (F-H) Analysis of anti-Aβ IgG subclasses in the sera of WT, nude, and TCR−/− mice receiving Aβ-peptide liposomes. Analysis of IgG subclasses were done on sera prepared from blood on day 28. Results are expressed as mean + SD OD values at a dilution of 1/400 (n = 6 per group).
A strong and persistent anti-Aβ IgG response in athymic nude mice and in TCR-deficient mice lacking both αβ and γδ T cells further verified a TI-antibody response (Figure 2D). The anti-Aβ IgM titers were also comparable in WT, nude and TCR-deficient mice (Figure 2E). The predominance of the IgG2b subclass and TI-associated IgG3 in all 3 mice strains (Figure 2F-H) further supporting that class switching can occur in the absence of T cells.
Peptide-specific TI IgG responses do not require APC-mediated costimulation or antigen presentation
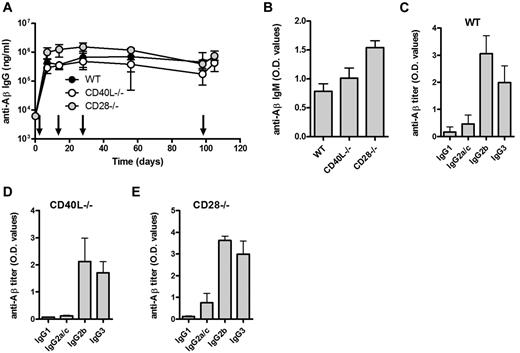
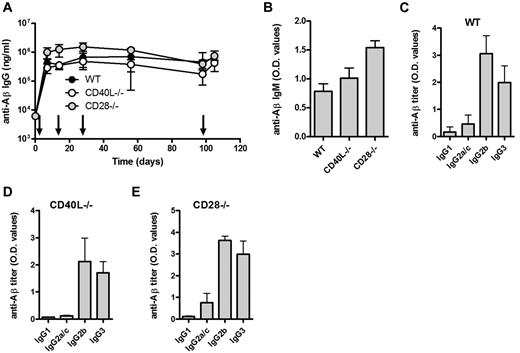
Although T cells were not required for the observed antibody response, costimulation of B cells may be provided by antigen-presenting cells (APCs). CD80/CD28, CD86/CD28, and CD40/CD40L interactions have been reported important for GC formation and class switching.14-16 We found that immunization with Aβ1-15 liposomes induced strong anti-Aβ IgM and IgG responses both in the CD28- and CD40L-deficient mice (Figure 3A-B). Again, predominance of IgG2b and TI-associated IgG3 similar to that observed in WT mice (Figure 3C-E).
Aβ-peptide liposomes induce IgG responses independent of CD40L and CD28. (A) Anti-Aβ IgG antibodies in the sera of WT, CD40L−/−, and CD28−/− mice receiving Aβ-peptide liposomes as indicated by arrows. Results are expressed as mean ± SD (n = 6 per group). (B) Analysis of anti-Aβ IgM and in the sera of WT, CD40L−/−, and CD28−/− mice from day 7. Results are expressed as mean OD values at a dilution of 1/100 + SD (n = 6 per group). (C-E) Analysis of anti-Aβ IgG subclasses in the sera of WT, CD40L−/−, and CD28−/− mice receiving Aβ-peptide liposomes. Analysis of IgG subclasses were done on sera prepared from blood on day 28. Results are expressed as mean OD values at a dilution of 1/400 + SD (n = 6 per group).
Aβ-peptide liposomes induce IgG responses independent of CD40L and CD28. (A) Anti-Aβ IgG antibodies in the sera of WT, CD40L−/−, and CD28−/− mice receiving Aβ-peptide liposomes as indicated by arrows. Results are expressed as mean ± SD (n = 6 per group). (B) Analysis of anti-Aβ IgM and in the sera of WT, CD40L−/−, and CD28−/− mice from day 7. Results are expressed as mean OD values at a dilution of 1/100 + SD (n = 6 per group). (C-E) Analysis of anti-Aβ IgG subclasses in the sera of WT, CD40L−/−, and CD28−/− mice receiving Aβ-peptide liposomes. Analysis of IgG subclasses were done on sera prepared from blood on day 28. Results are expressed as mean OD values at a dilution of 1/400 + SD (n = 6 per group).
To evaluate whether the observed TI antibody responses were dependent on the peptide amino acid sequence, a liposomal vaccine containing a Tau peptide was prepared (data not shown). Circular dichroism spectroscopy showed a typical β-sheet/β-strand conformation of the peptide when integrated into liposomes, similar to that of the Aβ1-15, and Thioflavin-T binding indicated that the Tau peptide was aggregated on the surface of the liposomes. Tau-peptide liposomes induced antigen-specific IgG responses in both WT and nude mice with similar IgG subclass distribution, which further suggests T-cell independency and underline the importance of the structural arrangement of antigen and adjuvant.
Aβ liposomes stimulates germinal center and somatic hypermutation with affinity maturation
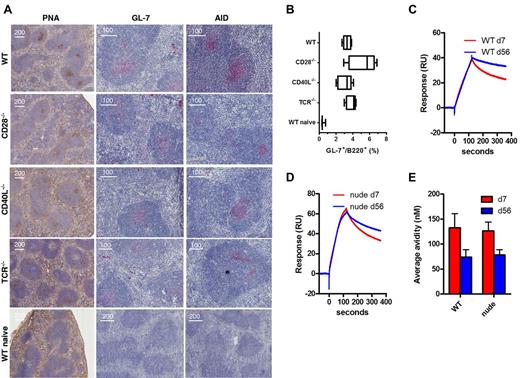
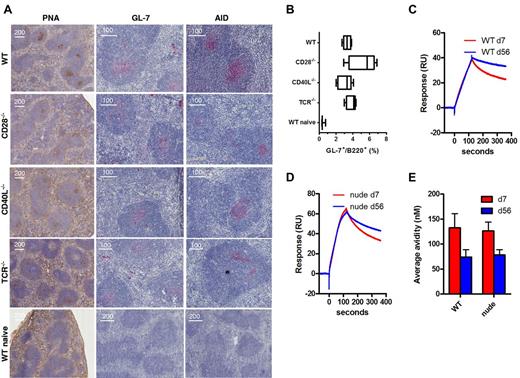
Immunization of WT mice with Aβ1-15 liposomes caused formation of GCs in both lymph node (not shown) and spleens (Figure 4A) by day 7 as shown histologically by PNA stainings in the follicles. The GCs were large and observed in almost every follicle. This was accompanied by increased expression of the GC marker GL-7 expression both histologically and as measured by flow cytometry (Figure 4B) as well as expression of activation-induced (cytidine) deaminase (AID), which mutates the Ig locus, thereby altering binding affinity for antigen (Figure 4A). Surprisingly, GC formation as well as the GL-7 and AID expression was also found after immunization of CD28-, CD40L-, and TCR-deficient mice (Figure 4A-B). In contrast, GC formation and GL-7/AID staining were not observed in naive mice. While evident that CD40L or CD28 were not required for GC formation and AID expression after immunization with Aβ1-15 liposomes, the magnitude and quality of such formation were slightly different. WT mice showed PNA-staining GCs in almost each follicle and the GCs were typically more distinct with sharp marginal zones. Deficient mice generally had lower number of GCs, and these were less distinct than those observed in WT mice. Especially in TCR-deficient mice, the GC formation was slower, being stronger on day 13 than day 7 postimmunization (data not shown).
TI germinal center formation, AID expression, and affinity maturation after immunization with Aβ-peptide liposomes. (A) WT C57BL/6, CD28−/−, CD40L−/−, and TCR−/− mice (n = 5) were immunized with MPLA-containing Aβ1-15 liposomes, and on day 7, spleens were analyzed for germinal center formation by histologic staining with (left panel) PNA, or antibodies against (middle panel) GL-7 or (right panel) AID. (B) GL-7 expression on B220 B cells was also analyzed by flow cytometry after dumping of splenocytes staining positive for IgM, IgD, CD4, CD8, Gr-1, CD11b, and CD11c. Analysis by (C-D) SPR/Biacore and (E) competitive ELISA of average avidity of antibodies in day 7 and day 56 sera from WT and nude mice immunized with Aβ-peptide liposomes.
TI germinal center formation, AID expression, and affinity maturation after immunization with Aβ-peptide liposomes. (A) WT C57BL/6, CD28−/−, CD40L−/−, and TCR−/− mice (n = 5) were immunized with MPLA-containing Aβ1-15 liposomes, and on day 7, spleens were analyzed for germinal center formation by histologic staining with (left panel) PNA, or antibodies against (middle panel) GL-7 or (right panel) AID. (B) GL-7 expression on B220 B cells was also analyzed by flow cytometry after dumping of splenocytes staining positive for IgM, IgD, CD4, CD8, Gr-1, CD11b, and CD11c. Analysis by (C-D) SPR/Biacore and (E) competitive ELISA of average avidity of antibodies in day 7 and day 56 sera from WT and nude mice immunized with Aβ-peptide liposomes.
The CDR regions of the heavy and light chains of 3 monoclonal antibodies generated after Aβ1-15 liposome vaccination were sequenced. Two antibodies each had one mutation in the heavy chains, suggesting that these antibodies have undergone affinity maturation (data not shown). To confirm this, we evaluated the avidity of the antibodies in sera from immunized WT and nude mice by SPR (Biacore) and competitive ELISA. By SPR, the dissociation of antibodies was slower in sera collected 56 days after immunization compared with sera from day 7 (Figure 4C-D). The relative dissociations were 0.59 (day 7) and 0.83 (day 56) for WT, or 0.50 (day 7) and 0.68 (day 56) for nude mice. Hence, the average avidity of the antibodies has increased over time in both WT and T cell–deficient nude mice. By competitive ELISA, the average avidity of the antibodies was also increased on day 56 compared with day 7 in both WT and nude mice (Figure 4E).
TLR4 and TRIF activation are required for TI anti-Aβ IgG responses stimulated by Aβ-peptide liposomes
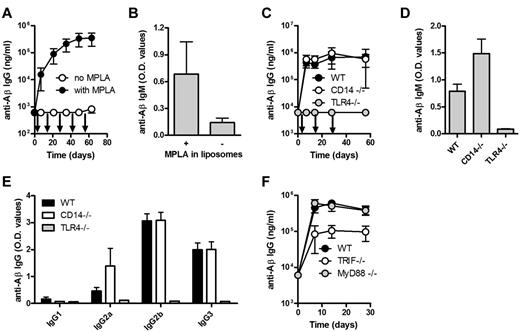
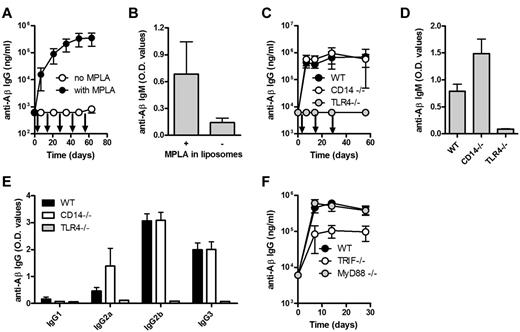
We assessed whether the repetitive structure of the antigen was sufficient for B-cell activation or whether a second signal was needed. While the liposomes containing both Aβ1-15 and MPLA stimulated strong TI anti-Aβ IgG responses, Aβ1-15 liposomes lacking MPLA did not trigger measurable anti-Aβ IgG (Figure 5A) or IgM (Figure 5B); MPLA liposomes lacking Aβ1-15 did not induce anti-Aβ IgG (data not shown). This clearly indicates the need for both antigen and MPLA stimulation.
TLR4 and TRIF are required for the induction of anti-Aβ IgG response by Aβ-peptide liposomes. (A) Anti-Aβ IgG and (B) IgM antibodies in the plasma of WT mice receiving Aβ-peptide liposomes (26 μg of PalmAβ1-15) with or without 30 μg of MPLA (as indicated by arrows). IgG results are expressed as mean ± SD, while IgM results are expressed as mean OD values at a dilution of 1/100 + SD (n = 10 per group). The data are representative of 2 independent experiments. (C) Anti-Aβ IgG and (D) IgM antibodies in the plasma of WT, CD14−/−, and TLR4−/− mice immunized s.c. with Aβ-peptide liposomes (as indicated by arrows). IgG results are expressed as mean ± SD, while IgM results from day 7 are expressed as mean OD at a dilution of 1/100 + SD (n = 6 per group). (E) Analysis of anti-Aβ IgG subclasses in the sera of WT mice, CD14−/− mice, and TLR4−/− mice receiving Aβ-peptide liposomes. Results are expressed as mean OD values at a dilution of 1/400 + SD (n = 6 per group). (F) Anti-Aβ IgG antibodies in the sera of WT mice, MyD88−/− mice, and TRIF−/− receiving Aβ-peptide liposomes (as indicated by arrows). IgG results are expressed as mean ± SD (n = 6 per group).
TLR4 and TRIF are required for the induction of anti-Aβ IgG response by Aβ-peptide liposomes. (A) Anti-Aβ IgG and (B) IgM antibodies in the plasma of WT mice receiving Aβ-peptide liposomes (26 μg of PalmAβ1-15) with or without 30 μg of MPLA (as indicated by arrows). IgG results are expressed as mean ± SD, while IgM results are expressed as mean OD values at a dilution of 1/100 + SD (n = 10 per group). The data are representative of 2 independent experiments. (C) Anti-Aβ IgG and (D) IgM antibodies in the plasma of WT, CD14−/−, and TLR4−/− mice immunized s.c. with Aβ-peptide liposomes (as indicated by arrows). IgG results are expressed as mean ± SD, while IgM results from day 7 are expressed as mean OD at a dilution of 1/100 + SD (n = 6 per group). (E) Analysis of anti-Aβ IgG subclasses in the sera of WT mice, CD14−/− mice, and TLR4−/− mice receiving Aβ-peptide liposomes. Results are expressed as mean OD values at a dilution of 1/400 + SD (n = 6 per group). (F) Anti-Aβ IgG antibodies in the sera of WT mice, MyD88−/− mice, and TRIF−/− receiving Aβ-peptide liposomes (as indicated by arrows). IgG results are expressed as mean ± SD (n = 6 per group).
MPLA may bind to either CD14 or the TLR4, both receptors for LPS. Antibody responses in CD14-deficient mice were similar to those measured in WT mice (Figure 5C). In TLR4-deficient mice, the anti-Aβ IgG (Figure 5C) and IgM (Figure 5D) responses were completely abrogated. The IgG subclass distribution in WT and CD14-deficient mice was comparable (Figure 5E).
TLR4 may signal through the adapter proteins MyD88 and/or TRIF. CD14-independent signaling by TLR4 has been shown to be dependent on MyD88 whereas CD14-dependent signaling activates both MyD88 and TRIF.17,18 Surprisingly, experiments in mice lacking either MyD88 or TRIF indicated that the antibody response was independent on MyD88, but significantly (P < .001) dependent on the activation of TRIF (Figure 5F).
TLR4 and TRIF activation in B lymphocytes is needed to induce a TI IgG response by Aβ1-15 liposomes
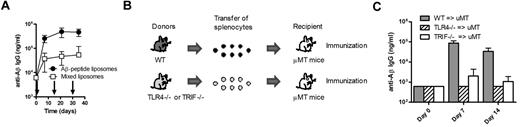
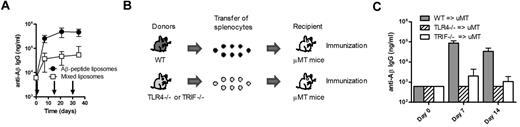
To determine whether antigen and TLR ligand activated the same cell, we injected mice with a mixture of 2 liposomal preparations, one containing the antigen Aβ1-15 and one containing only MPLA. This mixture significantly induced (P < .001) lower anti-Aβ IgG responses than the vaccine containing adjuvant and antigen on the same particles (Figure 6A), demonstrating that the antigen and the adjuvant should be in close proximity for induction of a TI IgG response. The data further suggest that the TLR4 signaling takes place directly on the B cells and not through mediation by APCs or other bystander cells. To verify the latter, mice specifically lacking TLR4 on B lymphocytes were used. TLR4-deficient or WT splenocytes were transferred into B cell–deficient μMT mice, which were then immunized with Aβ1-15 liposomes with MPLA (Figure 6B). While transfer of WT B cells expectedly enabled stimulation of anti-Aβ IgG responses in μMT mice, no response was observed in μMT mice that received TLR4-deficient B cells (Figure 6C). Thus, direct activation of TLR4 on B lymphocytes was critical for induction of Aβ-specific TI IgG responses. Similar experiments were done by transferring TRIF-deficient B cells into B cell–deficient μMT mice and then immunizing these with the MPLA-containing Aβ1-15 liposomes. The results revealed that the TRIF dependency observed above (Figure 5F) was associated with TRIF signaling directly on B cells because the antigen-specific IgG responses observed after transfer of WT B cells were abrogated in μMT that received TRIF-deficient cells (Figure 6C).
Triggering of TLR4 and TRIF in B lymphocytes is required for the induction of TI anti-Aβ IgG responses. (A) Anti-Aβ IgG antibodies in the sera of WT mice receiving liposomes containing both MPLA (19 μg of MPLA per dose) and Aβ1-15 (94 μg of PalmAβ1-15 per dose) or a 1:1 mixture of MPLA-containing liposomes and Aβ1-15–containing liposomes (as indicated by arrows). The 2 liposome formulations were mixed before injection. Results are expressed as mean ± SD (n = 10 per group). (B) Schematic representation of the generation of mice expressing or lacking TLR4 on B lymphocytes. Splenocytes from WT, TLR4−/−, or TRIF−/− mice were transferred into B cell–deficient μMT mice. (C) Analysis of anti-Aβ IgG responses in B cell–deficient μMT mice receiving splenocytes from WT, TLR4−/−, or TRIF−/− mice followed by immunization with Aβ-peptide liposomes. Results are expressed as mean + SD (n = 3 per group).
Triggering of TLR4 and TRIF in B lymphocytes is required for the induction of TI anti-Aβ IgG responses. (A) Anti-Aβ IgG antibodies in the sera of WT mice receiving liposomes containing both MPLA (19 μg of MPLA per dose) and Aβ1-15 (94 μg of PalmAβ1-15 per dose) or a 1:1 mixture of MPLA-containing liposomes and Aβ1-15–containing liposomes (as indicated by arrows). The 2 liposome formulations were mixed before injection. Results are expressed as mean ± SD (n = 10 per group). (B) Schematic representation of the generation of mice expressing or lacking TLR4 on B lymphocytes. Splenocytes from WT, TLR4−/−, or TRIF−/− mice were transferred into B cell–deficient μMT mice. (C) Analysis of anti-Aβ IgG responses in B cell–deficient μMT mice receiving splenocytes from WT, TLR4−/−, or TRIF−/− mice followed by immunization with Aβ-peptide liposomes. Results are expressed as mean + SD (n = 3 per group).
Discussion
The display of multiple copies of molecules on the surface of cells and microorganisms can induce antibody production without the requirement of T-helper cells when 10-20 or more B-cell receptors are stimulated simultaneously.19-21 Such semicrystalline arrays of antigens displayed on virus-like particles have been exploited to increase immunogenicity of antigens and to enable thymus-independent (TI) stimulation of B cells.6 However, in the absence of T help, antibody responses stimulated by nonreplicating protein antigens are characterized by short-lived IgM and no immunoglobulin class switch to IgG, IgA, or IgE.6 Similarly, antigen-specific TI IgG responses are detected in mice infected with live polyoma virus, but not in mice immunized with synthetic polyoma-like particles,7 suggesting that live and replicating organisms provide costimulatory signals crucial for the isotype switching. To our knowledge, the present study is the first to show TI IgG responses to a protein antigen. With a liposomal array of densely packed peptide and TLR4 ligand, long-lived IgG antibody responses were induced in several T cell–deficient mouse strains including athymic nude and TCR-deficient mice and in mice deficient in CD40L and CD28 signaling, both molecules which previously have been considered critical for the isotype switch and GC formation after immunization with protein-based antigens. Our data also suggest that the TI GC formation was associated with AID expression and affinity maturation. Although the degree of affinity maturation is difficult to estimate, the functionality of the antibodies induced by Aβ-peptide liposomes has been demonstrated previously by a decrease of Aβ species in the brain of immunized animals and a direct correlation of the antibody titers and improved cognition in an Alzheimer disease mouse model.8 The fact that the generated antibodies are neutralizing strongly suggest affinity maturation. One can also speculate that the speed of antibody production is also paralleled by very fast affinity maturation. That is, the affinity of the Aβ antibody is high already by day 7. Similar observations have been made for T cell–dependent B-cell responses induced by live vesicular stomatitis virus.22
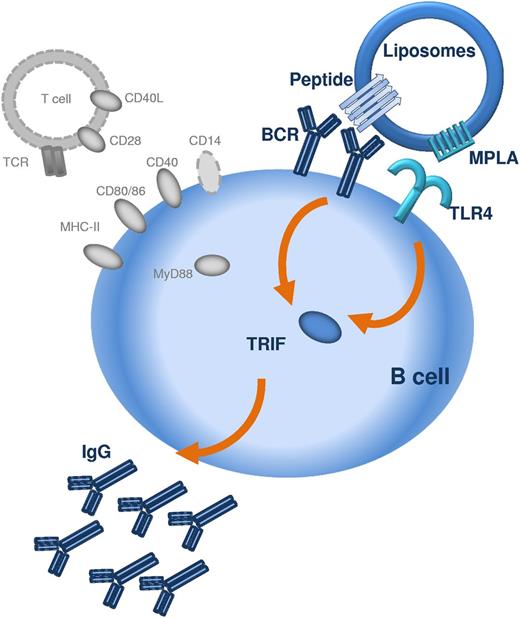
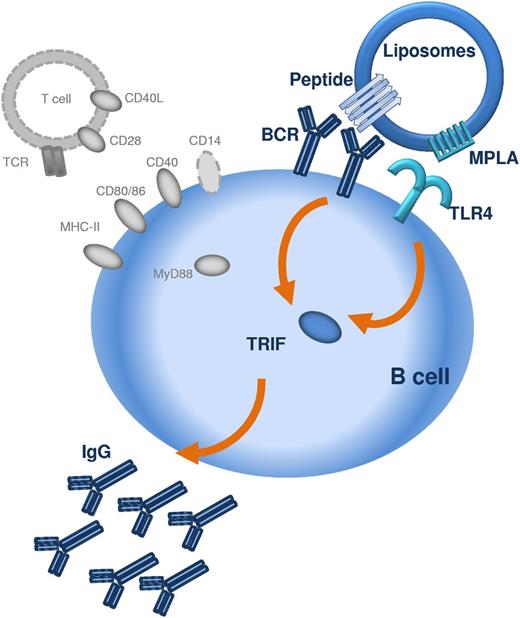
The repetitive display of peptide was not alone sufficient to induce antibody responses, but required the presence of a TLR ligand, here, MPLA. MPLA is a detoxified derivative of lipid A, derived from LPS of Gram-negative bacteria and has recently been approved for human use in cervical cancer, hepatitis, and influenza vaccines.23 MPLA mediates immune responses through activation of the TLR4/CD14/MD-2 coreceptor.24 In our study, stimulation of TRL4 with MPLA was necessary for the induction of both IgM and IgG responses, demonstrating that the TLR4 signaling is crucial for initial B-cell activation and not only for class-switch recombination, as recently suggested.25 Whether the stimulation of antibody responses requires the involvement of TLRs in general is currently a matter of debate,26-28 but it has become clear that TLR signaling can influence B-cell responses directly.29 In vitro, the direct stimulation of TLR425 or TLR929 on B cells has been shown to strengthen B-cell receptor signaling and immunoglobulin class switch. The requirement for direct triggering of TLR on B lymphocytes was recently demonstrated for TD antigens displayed on synthetic nanoparticles, where TLR4 and TLR7 ligands synergistically increased antigen-specific antibody responses.10 The current study suggest that the cross-linking of B-cell receptors for the stimulation of peptide-specific and thymus-independent antibody responses must involve the commitment of TLR4 on the B cells (Figure 7).
Schematic representation of the mechanism by which peptide liposomes can induce TI IgG responses. The peptide antigen is presented in an aggregated repetitive β-sheet conformation which allows crosslinking of several BCRs. Presentation of peptide antigen and TLR ligand (in this case MPLA) in close proximity, for example on liposomes, allows costimulation of BCR and TLR4 via TRIF on the same B lymphocyte. Activation of TRIF in B lymphocytes permits B-cell activation and class switching to IgG. T cells, MHC-II, CD40/CD40L, CD28/CD80, CD28/CD86, CD14, and MyD88 (all marked in gray) are not required for the induction of TI IgG responses by peptide liposomes.
Schematic representation of the mechanism by which peptide liposomes can induce TI IgG responses. The peptide antigen is presented in an aggregated repetitive β-sheet conformation which allows crosslinking of several BCRs. Presentation of peptide antigen and TLR ligand (in this case MPLA) in close proximity, for example on liposomes, allows costimulation of BCR and TLR4 via TRIF on the same B lymphocyte. Activation of TRIF in B lymphocytes permits B-cell activation and class switching to IgG. T cells, MHC-II, CD40/CD40L, CD28/CD80, CD28/CD86, CD14, and MyD88 (all marked in gray) are not required for the induction of TI IgG responses by peptide liposomes.
TLR4 is the only TLR identified until now that activates 2 signaling pathways, the MyD88-dependent and the TRIF-dependent pathways.30 MyD88 transmits signals culminating in NF-κB activation and production of inflammatory cytokines whereas TRIF activation leads to type I interferon production. The low toxicity of MPLA compared with LPS or lipid A has been associated with the preferential activation of TRIF and reduced MyD88 activation.31 A change from diphosphoryl (in LPS) to monophosphoryl (in MPLA) enabled TRIF-biased and MyD88-independent TLR4 signaling.32 More recently, the reduced MyD88 activation of MPLA was shown to be associated with impaired inflammasome priming and reduced caspase-1 activation and IL-1β production.33,34 Consistent with these studies, we found that the IgG secretion after immunization with MPLA-containing liposomes was impaired in TRIF-deficient, but not in MyD88-deficient B cells. Thus, the low toxicity of MPLA, associated with TRIF-biased signaling and reduced production of inflammatory cytokines, still allows for TI activation of B lymphocytes and robust IgG responses.
MyD88-independent LPS and lipid A signaling in macrophages have previously been shown to require CD14 expression.18 Surprisingly though, the TI IgG production after immunization with liposomes containing Aβ1-15 peptide and MPLA did not require TLR4 signal transduction through CD14 or MyD88. As mentioned in the paragraph above, the TRIF bias and MyD88 independency of MPLA compared with lipid A suggest that minor structural changes can have major impact on downstream signaling pathways. It is possible that these structural changes can explain the combined CD14 and MyD88 independency of MPLA as opposed to lipid A. The fact that CD14 is not expressed on B lymphocytes but primarily on myelomonocytic cells35 is further proof that antigen presentation does not take place by APCs such as DCs, macrophages, and monocytes, but that the peptide-MPLA assembly directly stimulate B-cell responses through ligation of B-cell receptors and TLR4.
Little is known about TLR4 signaling in B lymphocytes, and it remains to be determined whether TLR4 signaling pathways differ between B lymphocytes and macrophages, particularly with regards to the requirement for CD14, MyD88, and TRIF and how such differences may affect humoral responses. However, it has been suggested that TLR signaling in B cells is not required for induction of TI type II antibody IgG3 responses, and that such a response depended on TLR signaling in DCs and macrophages.28 The result of the present study demonstrate the contrary: TI IgG responses, including IgG3, strictly depended on TLR signaling in B cells and was fully independent on such signaling in professional APCs. By the same token, it was shown that class switching in B cells induced through transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) or TLR is MyD88 dependent.36 However, this was observed using the CpG as TLR agonist, and the study did not investigate the possibility of class switch recombination through other TLR signaling cascades. Our study demonstrated that class switch and GC formation can take place in the absence of MyD88 signals when the IgG response is TI and that TRIF and not MyD88 mediated such signaling directly on B cells.
The expression of TLR4 in mouse and human B cells differs and this needs to be considered for vaccination of human patients. While some studies show that human B cells do express TLR4,37 other studies suggest that TLR4 expression increases after TLR4 triggering. Indeed, TLR4 expression and function can be elevated in human B cells from patients with inflammatory diseases such as type 2 diabetes, periodontal disease, and Crohn disease.38 TLR4 expression has also been shown to be increased on PBMCs of Alzheimer disease patients.39 Reports also show that TLR4 signaling may have a regulating effect on the development of neuroinflammation and Alzheimer disease and activate TLR4 signaling,40 for which reason MPLA can expected to be a promising adjuvant in vaccines for Alzheimer disease.
As mentioned, both CD80/86 (on APCs) signaling to CD28 on T cells and CD40L (on T cells) signaling to CD40 on B cells have been considered crucial to providing isotype switch and maintenance of GCs.41 This may be true for T cell–dependent (TD) antigens, which do not meet a minimum requirement for CD40L, CD28, and TI class switching. MacLennan and coworkers showed that the CD28/CD40L restriction could be manipulated in a transgenic animal model with an abnormally high number of antigen-specific B cells and transgenic antigen receptors with a uniform high affinity for the TI type II hapten antigen (NP-Ficoll) that enabled isotype switch and formation of GCs.41 Our results suggest that such minimal requirement can be naturally overcome. Immunization with a dense assembly of Aβ peptide and MPLA allowed the delivery of sufficiently strong signals to the B-cell receptor with a potentially autocrine potentiation of the immune response. While previous reports have suggested that nonreplicating protein vaccines require T cells for antibody isotype switching,6,7 the present study demonstrates that a switch is feasible with a correct assembly of antigen and adjuvant. Independent of direct signaling or secondary bystander effects from T-helper cells, high titers of long-lasting IgG antibodies could be produced on immunization of mice with a densely packed array of peptide and MPLA on particles made up of phospholipids. One practical implication of this finding is the immunotherapeutic utilization in situations where T cells are less abundant because of disease (eg, AIDS), drug-mediated immune suppression (eg, organ transplant recipients and cancer patients), or where T-cell activation may cause immunopathology (eg, in Alzheimer disease). Indeed, immunization against Aβ1-42 in mice overexpressing the amyloid precursor protein decreased the number of plaques and improved behavior deficits, indicating that therapeutic Aβ vaccination may be beneficial against Alzheimer disease.42-47 However, clinical testing of Aβ1-42 vaccination revealed promising efficacy, but that 6% of treated patients developed encephalitis,48,49 which was attributed to a T-cell response against Aβ.50
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Davide Demurtas (Interdisciplinary Centre for Electron Microscopy, EPFL, Lausanne, Switzerland) for assistance with cryoelectron microscopy, Silvia Behnke, André Fitsche, and Ines Kleiber (University Hospital Zurich, Zurich, Switzerland) for immunohistochemistry, and Paolo Paganetti, Oskar Adolfsson, and Dairin Kieran (AC Immune SA) for critical review of the manuscript.
P.J. and T.M.K. (3100AO-122221/1) as well as Y.W.-M. (3200BO-118202) were supported by the Swiss National Science Foundation.
Authorship
Contribution: M.P. designed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; A.B.S. designed and performed research and collected data, analyzed, and interpreted data; R.M. and D.T.H. designed research and analyzed and interpreted data; V. Giriens, Y.W.-M., A.F., D.M.N., A.L.B., N.C., and P.R. performed research and collected data; M.P.L.-D. designed and performed research and analyzed and interpreted data; M.V., V. Gafner, and K.P. analyzed and interpreted data; A.P., T.M.K., and A.M. designed research; and P.J. designed and performed research, collected, analyzed, and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: M.P., A.B.S., R.M., V. Giriens, D.T.H., M.P.L.-D., D.M.N., M.V., A.L.B., V. Gafner, N.C., P.R., K.P., A.P., and A.M. were or are employees of AC Immune SA. The remaining authors declare no competing financial interests.
Correspondence: Andreas Muhs, AC Immune SA, PSE-B, Swiss Federal Institute of Technology (EPFL), 1015-Lausanne, Switzerland; e-mail: andreas.muhs@acimmune.com.
References
Author notes
A.M. and P.J. contributed equally to the study.