Abstract
Persistent high fever is one of the most typical clinical symptoms in dengue virus (DV)–infected patients. However, the source of endogenous pyrogen (eg, IL-1β) and the signaling cascade leading to the activation of inflammasome and caspase-1, which are essential for IL-1β and IL-18 secretion, during dengue infection have not been elucidated yet. Macrophages can be polarized into distinct phenotypes under the influence of GM-CSF or M-CSF, denoted as GM-Mφ and M-Mφ, respectively. We found that DV induced high levels of IL-1β and IL-18 from GM-Mφ (inflammatory macrophage) and caused cell death (pyroptosis), whereas M-Mφ (resting macrophage) did not produce IL-1β and IL-18 on DV infection even with lipopolysaccharide priming. This observation demonstrates the distinct responses of GM-Mφ and M-Mφ to DV infection. Moreover, up-regulation of pro-IL-1β, pro-IL-18, and NLRP3 associated with caspase-1 activation was observed in DV-infected GM-Mφ, whereas blockade of CLEC5A/MDL-1, a C-type lectin critical for dengue hemorrhagic fever and Japanese encephalitis virus infection, inhibits NLRP3 inflammasome activation and pyrotopsis in GM-Mφ. Thus, DV can activate NLRP3 inflammasome via CLEC5A, and GM-Mφ plays a more important role than M-Mφ in the pathogenesis of DV infection.
Key Points
The myeloid Syk-coupled C-type lectin 5A (CLEC5A) is critical for dengue virus–induced NALP3 inflammasome activation.
Inflammatory macrophage is distinct from resting macrophage in inflammasome activation by dengue virus and other pathogens.
Introduction
Dengue virus (DV) infection is one of the most prevalent arthropod-borne diseases in tropical and subtropical areas with approximately 50 million cases occurring annually.1,2 Even though most patients infected by DV are asymptomatic, DV infection can cause a wide spectrum of clinical symptoms, ranging from dengue fever (DF) to dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS). Clinical manifestation of DF includes fever, rash, headache, myalgia, arthralgia and retro-orbital pain, whereas plasma leakage, bleeding, thrombocytopenia, and hypovolemic shock are found in DHF/DSS.2-4 Even though secondary infection by DV with distinct serotype frequently correlates with severe DHF and DSS patients, a phenomenon known as antibody-dependent enhancement,4,5 primary infection occasionally causes severe illness, but the underlying mechanisms remain unclear.
Fever, one of the most typical symptoms for all DV patients, is caused by endogenous pyrogens (EPs).6 EPs are produced from stimulated leukocytes (or other cell types) once pathogens invade into the bloodstream. Among all EPs, IL-1β and TNF-α are 2 of the most important cytokines to induce fever in host.6,7 In addition to its fever-causing ability, IL-1β can regulate local and systemic inflammation by activating lymphocytes and promoting leukocytes infiltration to the inflammation site.8 Unlike TNF-α, the production of IL-1β requires the activation of dual pathways: the priming signals to induce the transcription and synthesis of pro-IL-1β, and the subsequent secondary signals to activate inflammasome and caspase-1.9 In addition to IL-1β, the secretion of IL-18 is also dependent on activation of inflammasome and caspase-1. Because serum levels of IL-1β and IL-18 correlate with the severity of dengue infection,10,11 these observations suggest that inflammasome activation may play critical roles in the pathogenesis of dengue infection. However, the mechanism by which DV activates inflammasomes to induce the secretion of IL-1β and IL-18 has not been well elucidated to date.
Macrophages (Mφ) are the major source of inflammatory cytokines,12 as well as the major target cells for DV replication.13,14 However, Mφ are heterogeneous throughout the body,15 and the phenotypic and functional diversities of Mφ are influenced by cytokines, which regulate their differentiation, tissue distribution, and defense to invaded pathogens.16 Among these mediators, M-CSF and GM-CSF are the most important factors that contribute to Mφ differentiation.17 In contrast to the ubiquitous and constant level of serum M-CSF,18 the expression of GM-CSF is spatially regulated and dramatically up-regulated at sites of inflammation or infection. This observation suggests that macrophage differentiation during inflammatory reactions is under the influence of GM-CSF, which can cause massive increase of macrophage population in spleen and induce splenomegaly.19 Based on these findings, Hamilton17 suggested that constant level of M-CSF is crucial to maintain Mφ population in a homeostatic and resting condition (denoted as resting Mφ), whereas the local and temporal increase of GM-CSF during inflammation polarizes Mφ to an inflammatory stage (denoted as inflammatory macrophage). Recently, an epidemiologic study demonstrated that elevated GM-CSF in the plasma of DV patients correlates with the severity of DF/DHF,11 suggesting that DV infection may switch macrophages from resting to inflammatory status in DV patients and affect the gravity of clinical outcomes.
Therefore, we investigate the roles of human macrophage subsets and signaling events involved in the production of IL-1β and IL-18. Here, we reported that DV replicates more efficiently in GM-Mφ than in M-Mφ. Moreover, higher levels of TNF-α, IL-1β, and IL-18 with lower level of IL-10 were found in GM-Mφ after DV infection. Furthermore, up-regulation of pro-IL-1β, pro-IL-18, and NLRP3 transcription with increased caspase-1 activity was found in DV-infected GM-Mφ. Interestingly, blockade of CLEC5A, a C-type lectin critical for DHF,20 and Japanese encephalitis virus infection,21 inhibits the activation of NLRP3 inflammasome, and abolishes DV-induced IL-1β production and pyrotopsis in GM-Mφ. These observations suggest that CLEC5A is critical to regulate NLRP3 inflammasome activation in DV infection and that the blockade of CLEC5A would be helpful to alleviate clinical symptoms in dengue patients.
Methods
Antibodies and reagents
The sources of antibodies for flow cytometry were purchased from manufacturers as follows: anti-CD163, anti–mannose receptor (MR), anti-DC-SIGN (BD Biosciences PharMingen); anti-CD14, anti–HLA-DR (BioLegend); anti-CD86 (eBioscience); anti–Toll-like receptor 3 (TLR3; Imgenex); anti-TLR7, anti-TLR8 (Abcam). The isotype-control mAbs were purchased from either BioLegend or BD Biosciences PharMingen. The sources of antibodies for blocking assay are as follows: antimannose receptor antibody (BioLegend); anti–DC-SIGN (R&D Systems); anti-CLEC5A blocking antibody.20 The antibodies for immunoprecipitation or immunoblotting are as follows: anti–IL-1β, anti–IL-18 (Santa Cruz Biotechnology), and antiactin antibody (Chemicon). Human monocytes were obtained from healthy donors at the Taipei Blood Center of Taiwan Blood Services Foundation, under a protocol (PM-99-TP-075) approved by the institutional review board of the Clinical Center of department of Health, Taiwan. Written informed consent was obtained from all donors in accordance with the Declaration of Helsinki.
The sources of inhibitors are as follows: caspase-1 inhibitor (Z-YVAD-FMK; R&D Systems); caspase-3 inhibitor (Ac-DEVD-CMK; Calbiochem); reactive oxygen species (ROS) inhibitor [(2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate, APDC; Alexis]; K+ channel inhibitor (glibenclamide; Tocris Bioscience); cathepsin B inhibitor (CA-074 Me; Calbiochem); Syk inhibitor [3-(1-methyl-1H-indol-3-yl-methylene)-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide; Calbiochem]. The sources of growth factors are as follows: human macrophage-colony stimulating factor (hM-CSF; R&D Systems); human granulocyte-macrophage colony-stimulating factor (hGM-CSF; Leucomax; Schering-Plough). All other chemical reagents, unless specified mentioned, were purchased from Sigma-Aldrich.
Cell cultures
Human monocyte–derived Mφ was cultured as previous described.22,23 CD14+ cells were purified from human peripheral leukocytes cells by high-gradient magnetic sorting using the VARIOMACS technique with anti-CD14 microbeads (Miltenyi Biotec). CD14+ monocytes were cultured in complete RPMI-1640 medium (Hyclone) supplemented with 10 ng/mL human M-CSF or 10 ng/mL human GM-CSF for 7 days at 37°C in 5% CO2.
RNA extraction and quantitative real-time PCR
Total cellular RNA from M-CSF-derived Mφ, GM-CSF–derived Mφ (5 × 106 cells), and other were extracted by using Trizol reagent (Invitrogen) according to the manufacturer's instructions. First-strand cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (Fermentas) as per the manufacturer's instructions. PCR reaction was performed in a LightCycler System SW 3.5.3 (Roche Applied Science) under the following conditions: PCR mixtures were denatured at 95°C for 5 minutes, followed by 40 cycles of 15 seconds at 95°C, 30 seconds at 58°C, and 30 seconds at 72°C for amplification. The mRNA expression level of each target gene was normalized to their respective GAPDH expression. The sequences of sense and antisense primers are shown in Table 1.
Flow cytometric analysis
To measure the expression of cell surface markers, differentiated Mφ were harvested and resuspended in FACS buffers (1% FCS in PBS), followed by incubation with fluorochrome-conjugated antigen-specific mAbs at 4°C for 30 minutes before washing twice and resuspended in PBS. To measure intracellular molecules expression, cells were fixed in 2% paraformaldehyde and permeabilized by adding 0.1% saponine subsequently before incubation with fluorochrome-conjugated antibodies for 30 minutes. Cells were then washed twice and resuspended in PBS, and samples were analyzed using a FACSCalibur cytometry (BD Biosciences).
Immunoprecipitation and immunoblotting
Mφ were primed with lipopolysaccharide (LPS, 5 ng/mL) for 4 hours, followed by incubation with DV (MOI = 30) for 2 hours at 37°C. At 24 hours after infection, cells were pelleted down and dissolved in lysis buffer (50mM Tris-HCl pH 8.0, 150mM NaCl, 1% NP40, protease inhibitors), followed by immunoprecipitation using anti–IL-18 antibody (Santa Cruz Biotechnology) conjugated to Protein A–Sepharose CL-4B (GE Healthcare Bio-Science AB) for 4-6 hours at 4°C. The immunocomplexes were fractionated on SDS-PAGE, followed by blotting onto polyvinylidene difluoride membranes. Blot was incubated with anti–IL-18 (Santa Cruz Biotechnology), followed by incubation with HRP-conjugated goat anti–rabbit IgG (Chemicon), and signals were visualized by the ECL-plus system (GE Healthcare). IL-1β in cell lysates was detected by Western blotting analysis directly without immunoprecipitation. Blot was incubated with anti–IL-1β antibody (Santa Cruz Biotechnology), followed by HRP-conjugated goat anti–rabbit IgG antibody (Chemicon). For internal control (actin), membrane was further stripped for 10 minutes by ReBlot Plus Strong Antibody Stripping Solution (Chemicon), and were reprobed with antiactin antibody (Chemicon) and HRP-conjugated goat anti–mouse IgG antibody (Chemicon) subsequently.
Preparation of DV
Preparation of DV strain (strain PL046, serotype 2) and determination of viral titers were described previously.24 Viral titers were determined by plaque assay using baby hamster kidney (BHK-21) cells. The plaque numbers were counted and the results were shown as PFU per milliliter (PFU/mL).
DV infection to macrophages
Mφ (3 × 105/mL) were incubated with C6/36 culture medium (mock control) or DV of various MOI at 37°C for 2 hours. Alternatively, cells were primed with LPS (5 ng/mL) for 4 hours before incubation with DV or C6/36 culture medium. The unabsorbed viruses were removed by washing once with serum-free RPMI, and cells were further cultured with fresh complete RPMI (10% FCS) for various time periods. Supernatants from DV-infected Mφ were harvested at the indicated time points, and infectious viral particles in the supernatants were determined by plaque assay using BHK-21 cells. The adherent cells were lysed with 0.1% Triton for 30 minutes at the indicated time points, and intracellular cytokines were detected by ELISA.
Immunofluorescence analysis for DV antigens
Macrophages were seeded on a polylysine-coated slide and infected with DV virus as described in “DV infection to macrophages.” At 48 hours after infection, macrophages were washed in PBS and fixed in 2% paraformaldehyde for 30 minutes. Intracellular viral antigens were detected by incubating anti-nonstructural protein 3 (NS3) mAb (clone 3–274) and Cy3-conjugated donkey anti–mouse IgG antibodies (Jackson ImmunoResearch Laboratories) subsequently, whereas nuclei were counterstained with Hoechst 33342 (Invitrogen). The signal of DV antigen were observed under a confocal microscope (Olympus FV1000).
ELISA
The amounts of cytokines and cleaved caspase-1 (p20) in the culture supernatants were determined by ELISA. TNF-α, IL-1β, IL-10, and caspase-1 (p20) were purchased from R&D Systems, whereas IL-18 and IFN-α were purchased from Bender MedSystems.
Trypan blue staining
The viability of DV-infected macrophages was determined by incubating cells with 0.4% Trypan blue in PBS for 10 minutes at room temperature and observed under a light microscope (DIAPHOT 300; Nikon) with a Evolution VF COOLED COLOR digital camera (MediaCybernetics).
LDH release assay
Cells grown in 24-well microtitration plates were incubated with DV (MOI = 5 or 30), and supernatants were harvested at the indicated time points to determine the amount of cytosolic lactate dehydrogenase (LDH) by using the CytoTox 96 nonradioactive cytotoxicity assay kit according to the manufacturer's instruction (Promega). Maximum LDH release was determined after cell was lysed with Triton X-100. The percentages of cytotoxicity after DV infection were calculated as (experimental release-culture medium background)/(Triton X-100 release-culture medium background) × 100.
TUNEL assay
Cells grown in 6-cm2 dishes were incubated with DV (MOI = 5 and 30) and were harvested at the indicated time points to examine the apoptotic cells using the APO-DIRECT flow cytometry kit (Chemicon) according to the manufacturer's instructions. The percentages of positive signal were compared with the basal level of mock group by TUNEL assay.
Blocking assay
Macrophages grown in 96-well microtitration plate (6 × 104/0.2mL) were primed with LPS (5 ng/mL) for 4 hours, followed by preincubation with CLEC5A-, MR-, or DC-SIGN-blocking antibody (0.1-10 μg/well) for 1 hour, before incubation with DV (MOI = 5 or 30) for 2 hours at 37°C. Cells were then washed with serum-free RPMI once and incubated in complete medium (RPMI with 10% FCS) before harvesting supernatants to determine cytokines contents by ELISA. Alternatively, cells were preincubated with various inhibitors for 60 minutes before incubating with DV (MOI = 5 or 30) for 2 hours at 37°C.
Endothelial cell permeability assay
Permeability change of human dermal microvascular endothelial cells (HMEC-1) monolayers was determined by measuring the passage of HRP after incubation with supernatants of DV-infected macrophages. Briefly, HMEC-1 (2 × 105) were seeded in 24-well collagen and fibronectin-coated transwell (6.5-nm diameter, 0.4-μm pore size, Corning Life Sciences) and cultured in the Complete Medium 200 (Cascade Biologics) with low serum growth supplement. On day 2 after plating, culture media were replaced with mock or DV-infected Mφ culture supernatants and incubated for 6 hours. HRP (0.5 μg in 10 μL) was added to the upper chamber of transwell for 15 minutes before harvesting medium from lower chamber (10 μL) to determine HRP activity by incubating with TMB (3,3′,5,5′-tetramethylbenzidine) for 20 minutes, and analyzed by spectrophotometer at OD450 nm after adding sulfuric acid to stop reaction.
RNA interference (siRNA) assay
GM-Mφ grown in 96-well microtitration plate were transfected with 100nM nontargeting control siRNA (AllStars Negative Control siRNA, QIAGEN), or with 100nM of NLRP3 siRNA (HS_CIAS1_6, QIAGEN) by HiPerFect Transfection Reagent (QIAGEN) according to the manufacturer's instructions. At 24 hours after transfection, GM-Mφ were primed with LPS (5 ng/mL) for 4 hours, followed by incubation with DV (MOI = 5 or 30) for 2 hours at 37°C. Cells were washed with serum-free RPMI once and incubated in complete medium (RPMI with 10% FCS) for 24 hours before harvesting supernatants to determine cytokines contents by ELISA.
Statistical analysis
Values are expressed as mean ± SEM. All experiments were evaluated by Student t test from the Prism software package (GraphPad Version 5.00), and a 2-tailed P value of < .05 was considered significant.
Results
Differential phenotypes of GM-Mφ and M-Mφ
To obtain inflammatory (GM-Mφ) and resting (M-Mφ) macrophages, human CD14+ monocytes were incubated with GM-CSF and M-CSF, respectively, in vitro for 7 days. GM-Mφ appears as fried-egg shape with relatively abundant cytoplasm and condensed nucleus (supplemental Figure 1A-B left panels, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), whereas M-Mφ has smaller nucleus with abundant vacuoles in the cytoplasm (supplemental Figure 1A-B right panels). In addition, the cytoplasm of GM-Mφ is rich in mitochondria, whereas the cytoplasm of M-Mφ is filled with lysosomes (supplemental Figure 1C).
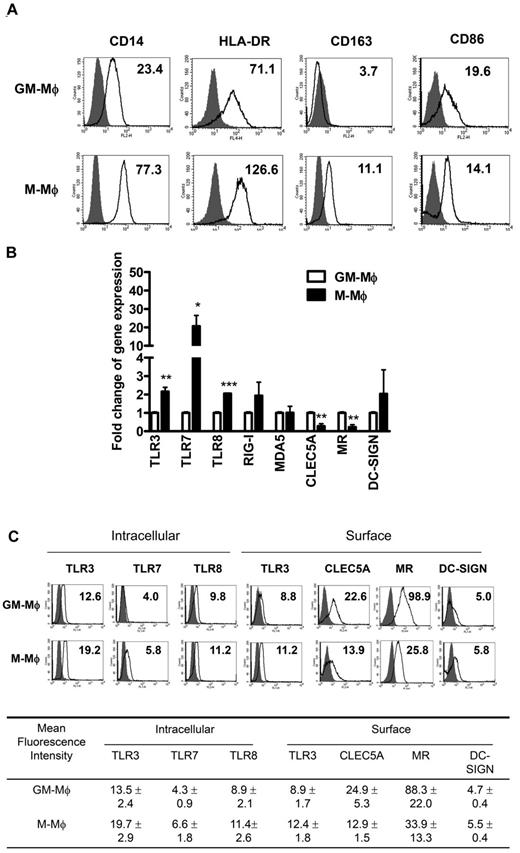
Flow cytometric analysis shows the differential expression of CD14, HLA-DR, CD163, and CD86 between M-Mφ and GM-Mφ (Figure 1A), and this observation is in accord with previous report that GM-Mφ express lower levels of CD163.25 We further examined the expression of pattern recognition receptors involved in DV-induced inflammatory cytokine releases, such as Toll-like receptors (TLRs),26-28 cytosolic retinoid acid-inducible gene I (RIG-I)–like receptors,28 and C-type lectin receptors (CLRs)20,29,30 by real-time PCR (Figure 1B) and flow cytometry (Figure 1C). Compared with M-Mφ, GM-Mφ displays higher levels of CLEC5A and MR/CD206, with lower levels of TLR3, TLR7, TLR8, and RIG-I. The differential expression of these innate immunity receptors and sensors in M-Mφ and GM-Mφ suggests a further functional difference that may cause distinct responses during DV infection. The mean fluorescence intensity of DV-pattern recognition receptor expression on GM-Mφ and M-Mφ is shown in bottom panel of Figure 1C.
Expression of DV-recognizing pattern-recognition receptors and sensors in GM-Mφ and M-Mφ. (A) Surface markers in GM-Mφ and M-Mφ were examined by flow cytometry. Gray area represents isotype control; and solid lines, mAb staining. The numbers in the figure show the mean fluorescence intensity (MFI) of mAb staining. (B) TLRs, intracellular sensors (RIG-I, MDA-5), and C-type lectins (CLEC5A, MR, and DC-SIGN) gene expression in GM-Mφ and M-Mφ were examined by real-time PCR and normalized by their comparative internal control, GAPDH. Fold change of gene expression in the y-axis was calculated based on the same gene expression in GM-Mφ. Data are mean ± SEM from 3 independent experiments. *P < .05. **P < .01. ***P < .001 (all P values Student t tests). (C) Expression of TLRs and CLRs in GM-Mφ and M-Mφ was examined by flow cytometry. The expression of surface TLR3, CLRs, and intracellular TLRs (TLR3, TLR7, and TLR8) was detected by flow cytometry. Gray area represents isotype control; and solid lines, mAb staining. The numbers in the figure show the MFI of mAb staining (top panel). The MFI of TLRs and CLRs was determined using Cellquest Version 3.3 software and was expressed as the mean ± SEM from 3 independent experiments (bottom panel).
Expression of DV-recognizing pattern-recognition receptors and sensors in GM-Mφ and M-Mφ. (A) Surface markers in GM-Mφ and M-Mφ were examined by flow cytometry. Gray area represents isotype control; and solid lines, mAb staining. The numbers in the figure show the mean fluorescence intensity (MFI) of mAb staining. (B) TLRs, intracellular sensors (RIG-I, MDA-5), and C-type lectins (CLEC5A, MR, and DC-SIGN) gene expression in GM-Mφ and M-Mφ were examined by real-time PCR and normalized by their comparative internal control, GAPDH. Fold change of gene expression in the y-axis was calculated based on the same gene expression in GM-Mφ. Data are mean ± SEM from 3 independent experiments. *P < .05. **P < .01. ***P < .001 (all P values Student t tests). (C) Expression of TLRs and CLRs in GM-Mφ and M-Mφ was examined by flow cytometry. The expression of surface TLR3, CLRs, and intracellular TLRs (TLR3, TLR7, and TLR8) was detected by flow cytometry. Gray area represents isotype control; and solid lines, mAb staining. The numbers in the figure show the MFI of mAb staining (top panel). The MFI of TLRs and CLRs was determined using Cellquest Version 3.3 software and was expressed as the mean ± SEM from 3 independent experiments (bottom panel).
GM-Mφ is more susceptible to DV infection than M-Mφ
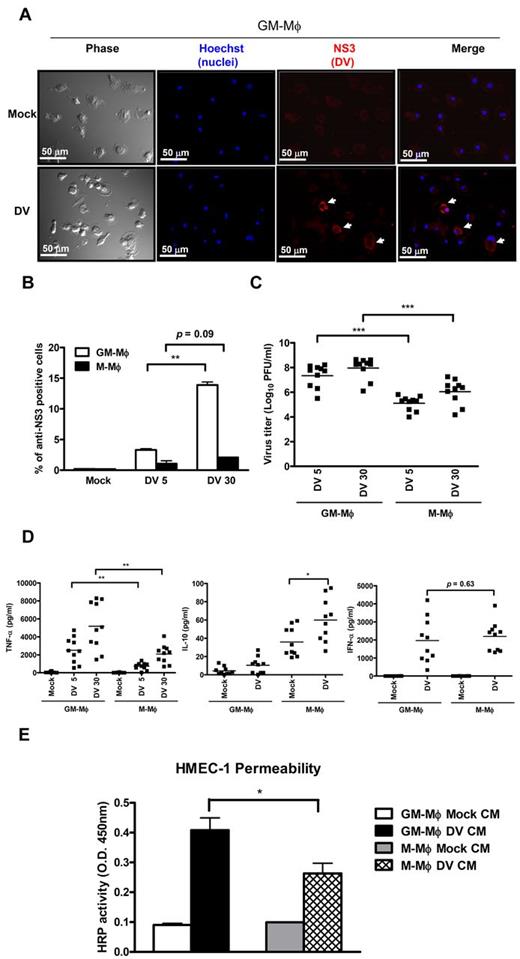
Previously, we have shown that DV can replicate in M-Mφ and induce TNF-α production,20 but whether DV can infect and replicate in GM-Mφ is not investigated yet. When the susceptibility of GM-Mφ to DV infection was examined by the expression of NS3 protein using immunofluorescence (IF), we identified intensive NS3-positive signals (Figure 2A) and a higher percentage of NS3-positive cells after incubation with DV (Figure 2B). The higher infection rate in GM-Mφ correlates with the higher expression of MR, which is shown to facilitate DV infection to macrophage.30 Moreover, GM-Mφ released higher amount of DV (100-fold higher) than M-Mφ as determined by plaque assay (Figure 2C), and the kinetics of virus titers after DV infection were shown in supplemental Figure 2. Furthermore, higher amounts of TNF-α with lower level of IL-10 were detected in DV-infected GM-Mφ (P < .05). However, there was no significant difference in IFN-α production between DV-infected GM-Mφ and M-Mφ (P = .63; Figure 2D). Furthermore, supernatant from DV-infected GM-Mφ is more potent than that of DV-infected M-Mφ to increase endothelial permeability by in vitro assay (P < .05; Figure 2E). All of these observations suggest that GM-Mφ appears to play more important role than M-Mφ in the pathogenesis of DV infection.
Differential responses of GM-Mφ and M-Mφ to DV infection. (A) Expression of NS3 in DV-infected GM-Mφ. GM-Mφ were infected with mock (C6/36 culture medium) or DV (MOI = 30) for 2 hours at 37°C. At 48 hours after infection, NS3 signals were detected by immunofluorescence staining. First column represents phase; second column, Hoechst 33342 staining; third column, NS3; and fourth column, merge of Hoechst 33342 and NS3. White arrows indicate NS3 in DV-infected cells. Scale bar represents 50 μm. (B) Percentage of NS3-positive cells. DV-infected GM-Mφ and M-Mφ were subjected to immune staining to determine the percentage of NS3-positive cells. Data are the mean ± SEM from 3 independent experiments. **P < .01 (Student t test). (C) Supernatants from DV-infected GM-Mφ and M-Mφ were subjected to plaque assay as described in “Preparation of DV” to determine DV titers. ***P < .001 (Student t test). (D) Cytokines secreted from DV-infected Mφ were determined by ELISA. *P < .05 (Student t test). **P < .01 (Student t test). (E) Permeability change of endothelial cell. HMEC-1 cells grown on the transwell membranes were incubated with supernatants from mock-infected or DV-infected Mφ for 6 hours, then determined permeability change by measuring passed HRP in the lower chamber. Data are the mean ± SEM from 3 independent experiments. *P < .05 (Student t test). Each dot represents an individual donor; and horizontal bars represent mean values for each group. DV5 30 indicates MOI = 5 and 30, respectively; and CM, conditional medium.
Differential responses of GM-Mφ and M-Mφ to DV infection. (A) Expression of NS3 in DV-infected GM-Mφ. GM-Mφ were infected with mock (C6/36 culture medium) or DV (MOI = 30) for 2 hours at 37°C. At 48 hours after infection, NS3 signals were detected by immunofluorescence staining. First column represents phase; second column, Hoechst 33342 staining; third column, NS3; and fourth column, merge of Hoechst 33342 and NS3. White arrows indicate NS3 in DV-infected cells. Scale bar represents 50 μm. (B) Percentage of NS3-positive cells. DV-infected GM-Mφ and M-Mφ were subjected to immune staining to determine the percentage of NS3-positive cells. Data are the mean ± SEM from 3 independent experiments. **P < .01 (Student t test). (C) Supernatants from DV-infected GM-Mφ and M-Mφ were subjected to plaque assay as described in “Preparation of DV” to determine DV titers. ***P < .001 (Student t test). (D) Cytokines secreted from DV-infected Mφ were determined by ELISA. *P < .05 (Student t test). **P < .01 (Student t test). (E) Permeability change of endothelial cell. HMEC-1 cells grown on the transwell membranes were incubated with supernatants from mock-infected or DV-infected Mφ for 6 hours, then determined permeability change by measuring passed HRP in the lower chamber. Data are the mean ± SEM from 3 independent experiments. *P < .05 (Student t test). Each dot represents an individual donor; and horizontal bars represent mean values for each group. DV5 30 indicates MOI = 5 and 30, respectively; and CM, conditional medium.
LPS enhances IL-1β and IL-18 secretion in DV-infected GM-Mφ
We further investigated whether DV can activate inflammasomes to induce the secretion of IL-1β and IL-18 in GM-Mφ and M-Mφ. To address this question, supernatants from DV-infected GM-Mφ and M-Mφ were harvested to determine cytokine levels by ELISA. Although IL-1β and IL-18 levels were not detectable in DV-infected M-Mφ with or without LPS priming (supplemental Figure 3), high amounts of IL-18 (2500 pg/mL at MOI = 30) with low amounts of IL-1β (50 pg/mL at MOI = 30) were detected in DV-infected GM-Mφ at 48 hours after infection (Figure 3A). After LPS priming, intracellular IL-1β was dramatically increased (4000 pg/mL at MOI = 30) at 6 hours and decreased gradually to basal level at 24 hours. The decrease of intracellular IL-1β associated with increase of secreted IL-1β in supernatant, and IL-1β level reached peak at 24 hours after infection (Figure 3B top panel). This indicates that DV infection can enhance IL-1β secretion from LPS-primed GM-Mφ. In contrast, LPS priming did not further enhance IL-18 secretion under the same condition (Figure 3B bottom panel).
LPS priming enhanced DV-induced IL-1β and IL-18 processing in GM-Mφ. (A) Secretion of IL-1β and IL-18 from DV-infected GM-Mφ. Cytokine levels in DV-infected Mφ were harvested and determined by ELISA. Each dot represents an individual donor; horizontal bars represent mean values for each group. ***P < .001 (Student t test). (B-C) LPS priming enhanced IL-1β secretion and pro-IL-1β processing from DV-infected GM-Mφ. GM-Mφ were primed with LPS (5 ng/mL) or without priming for 4 hours, followed by incubation with DV (MOI = 5 and 30) or mock (C6/36 culture medium) for 2 hours at 37°C. Cytokine levels in the culture supernatants and cell lysates were determined by ELISA at various time points after infection (B), whereas mature and immature forms of IL-1β and IL-18 in cell lysates were harvested at 24 hours after infection and determined by Western blot (C). Sup. indicates supernatant.
LPS priming enhanced DV-induced IL-1β and IL-18 processing in GM-Mφ. (A) Secretion of IL-1β and IL-18 from DV-infected GM-Mφ. Cytokine levels in DV-infected Mφ were harvested and determined by ELISA. Each dot represents an individual donor; horizontal bars represent mean values for each group. ***P < .001 (Student t test). (B-C) LPS priming enhanced IL-1β secretion and pro-IL-1β processing from DV-infected GM-Mφ. GM-Mφ were primed with LPS (5 ng/mL) or without priming for 4 hours, followed by incubation with DV (MOI = 5 and 30) or mock (C6/36 culture medium) for 2 hours at 37°C. Cytokine levels in the culture supernatants and cell lysates were determined by ELISA at various time points after infection (B), whereas mature and immature forms of IL-1β and IL-18 in cell lysates were harvested at 24 hours after infection and determined by Western blot (C). Sup. indicates supernatant.
Western blot analysis showed that both pro-IL-1β and pro-IL-18 were constitutively expressed in GM-Mφ (Figure 3C left panels), and LPS priming can further increase pro-IL-1β, but not pro-IL-18 (Figure 3C right panels). Moreover, mature IL-1β and IL-18 were up-regulated in DV-infected, LPS-primed GM-Mφ (Figure 3C).
The expression of IL-1α is similar to IL-1β, even though the amount after LPS priming was 30-fold less than IL-1β (50 pg/mL and 1500 pg/mL; supplemental Figure 4 left panel). Because IL-1 receptor antagonist (IL-1Ra) opposed the production of free IL-1β,8 we also determined IL-1Ra amount in supernatant and cell lysate by ELISA. We found that DV alone can trigger the production of IL-1Ra (40 ng/mL), whereas LPS priming has mild effect to enhance IL-1Ra production (75 ng/mL) after DV infection (supplemental Figure 4 right panel). Thus, we conclude that LPS priming is essential for the production of mature IL-1α/β in DV-infected GM-Mφ, whereas DV alone is sufficient to produce mature IL-18 and IL-1Ra. Because excessive IL-1Ra (100- to 1000-fold) is necessary to neutralize IL-1β activity,31,32 the abundant IL-1β (1500 pg/mL) after LPS priming will cause fever and induce severe inflammation.
Caspase-1 inhibitor suppresses IL-18 secretion and pyroptosis in GM-Mφ
In addition to producing IL-1 and IL-18, DV-infected GM-Mφ became trypan blue-positive at 48 hours after infection (Figure 4A). Increase of LDH release (Figure 4B) and DNA fragmentation (Figure 4C) were also observed under the same condition. Because caspase-1 activation is responsible for pyroptosis33 and the secretion of IL-1β and IL-18,9 we detected p20 (activated caspase-1) in the supernatants of DV-infected GM-Mφ by ELISA. Caspase-1 p20 was detectable at 6 hours (20 pg/mL at MOI = 30) and reached peak at 24 hours (120 pg/mL) post infection (Figure 4D). In addition, caspase-1 inhibitor Z-YVAD-FMK was able to suppress the secretion of IL-1β and IL-18 (supplemental Figure 5; Figure 4E) and LDH release (Figure 4F), whereas caspase-3 inhibitor has no effect under the same condition. This observation suggests that DV activates caspase-1 to secrete IL-1β and IL-18, and induces GM-Mφ pyroptosis.
DV infection activated caspase-1 and induces pyroptosis. (A) GM-Mφ or M-Mφ was infected with DV (MOI = 5 and 30) as described in “DV infection to macrophages.” At 48 hours after infection, cells were stained with trypan blue and observed under a light microscope. Bar represents 100 μm. (B) LDH released from DV-infected GM-Mφ. The amounts of LDH released in the supernatants were determined as described in “LDH release assay.” Values represent mean ± SEM of triplicate independent experiments. **P < .01; ***P < .001 (Student t tests). (C) TUNEL assay. DNA damage in DV-infected GM-Mφ was determined by TUNEL assay as described in “TUNEL assay.” M1 indicates percentage of TUNEL-positive cells. (D) The amounts of caspase-1 p20 released from mock or DV-infected GM-Mφ were determined by ELISA. (E-F) Inhibition of DV-induced IL-18 production (E) and cell death (F) by caspase-1 inhibitor. GM-Mφ were preincubated with DMSO, caspase-1 inhibitor (50μM), and caspase-3 inhibitor (50μM), and then infected with DV (MOI = 30) as described in “Blocking assay.” At 48 hours after infection, supernatants were subjected to ELISA assay to determine IL-18 levels and LDH amounts. Data are the mean ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001 (Student t tests). DV5, 30 indicates MOI = 5 and 30, respectively; and ND, not detectable.
DV infection activated caspase-1 and induces pyroptosis. (A) GM-Mφ or M-Mφ was infected with DV (MOI = 5 and 30) as described in “DV infection to macrophages.” At 48 hours after infection, cells were stained with trypan blue and observed under a light microscope. Bar represents 100 μm. (B) LDH released from DV-infected GM-Mφ. The amounts of LDH released in the supernatants were determined as described in “LDH release assay.” Values represent mean ± SEM of triplicate independent experiments. **P < .01; ***P < .001 (Student t tests). (C) TUNEL assay. DNA damage in DV-infected GM-Mφ was determined by TUNEL assay as described in “TUNEL assay.” M1 indicates percentage of TUNEL-positive cells. (D) The amounts of caspase-1 p20 released from mock or DV-infected GM-Mφ were determined by ELISA. (E-F) Inhibition of DV-induced IL-18 production (E) and cell death (F) by caspase-1 inhibitor. GM-Mφ were preincubated with DMSO, caspase-1 inhibitor (50μM), and caspase-3 inhibitor (50μM), and then infected with DV (MOI = 30) as described in “Blocking assay.” At 48 hours after infection, supernatants were subjected to ELISA assay to determine IL-18 levels and LDH amounts. Data are the mean ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001 (Student t tests). DV5, 30 indicates MOI = 5 and 30, respectively; and ND, not detectable.
DV activates NLRP3 inflammasome in GM-Mφ
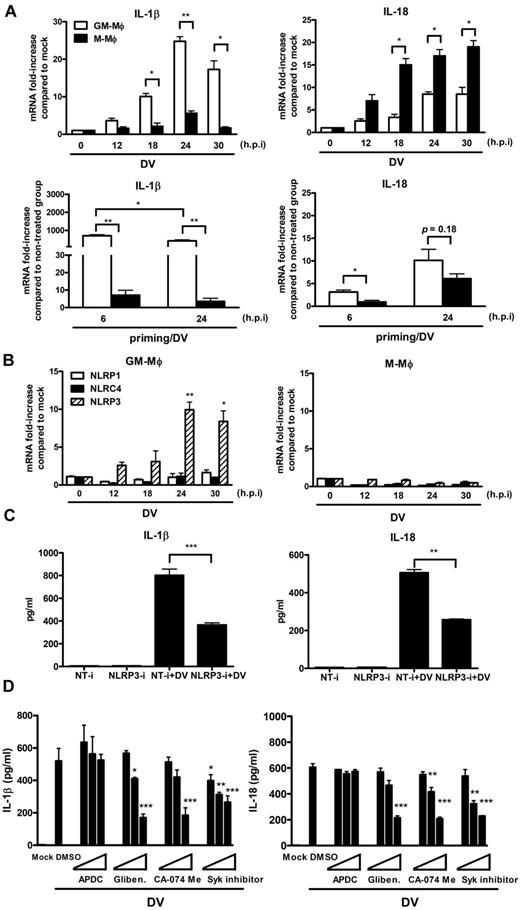
We further investigated whether DV infection up-regulated the transcription of cytokines (IL-1β and IL-18) and NLRs (NLRP1, NLRC4, and NLRP3), which were essential for the activation of inflammasomes in macrophages.34 We found that DV differentially up-regulated the transcription of IL-1β and IL-18 in M-Mφ and GM-Mφ, when cells were either incubated with DV directly (Figure 5A top panels), or primed with LPS before DV infection (Figure 5A bottom panels). In contrast to GM-Mφ, less IL-1β and more IL-18 transcripts were induced in M-Mφ when cells were incubated with DV directly (Figure 5A top panels). Compared with direct DV incubation, LPS priming dramatically enhanced DV-induced IL-1β transcription (20-fold vs 900-fold at 24 hours after infection) in GM-Mφ, whereas LPS priming has mild effect on IL-18 transcription. In contrast, LPS priming did not affect the transcription of IL-1β, whereas it suppresses IL-18 transcription (15-fold vs 5-fold at 24 hours after infection) in M-Mφ (Figure 5A bottom panels). These results are in accord with the observation that LPS priming only enhances IL-1β secretion (Figure 3B top panels), whereas it only has mild effect in IL-18 secretion in GM-Mφ (Figure 3B bottom panels).
DV-induced IL-1β/IL-18 secretion is dependent on NLRP3 inflammasome activation in GM-Mφ. (A) Determination of cytokine mRNA levels by real-time PCR. GM-Mφ or M-Mφ were either incubated with DV directly (top panels) or primed with LPS (5 ng/mL) for 4 hours before incubation with DV (MOI = 5) for 2 hours at 37°C. Total RNAs from mock or DV-infected GM-Mφ and M-Mφ were isolated for reverse-transcription into cDNA at the indicated time point after infection. The expression levels was determined by real-time PCR and normalized with GAPDH. Fold change of gene expression in the y-axis was calculated based on the same gene expression in mock group in DV-infected macrophages (top panels), or based on the same gene expression in nontreated group (bottom panels). Data are the mean ± SEM from 3 independent experiments. *P < .05; **P < .01 (Student t tests). h.p.i indicates hours after infection. (B) Determination of NLR mRNAs by real-time PCR. The expression levels of NLRs after DV infection in both macrophages were determined by real-time PCR and normalized with GAPDH. Fold change of gene expression in the y-axis was calculated based on the same gene expression in mock group. Data are the mean ± SEM from 3 independent experiments. *P < .05; **P < .01 (Student t tests). (C) Knockdown NLRP3 by siRNA. LPS (5 ng/mL)–primed GM-Mφ were transfected with the nontargeting siRNA control (NT-i) or the NLRP3 siRNAs (NLRP3-i) as described in “RNA interference (siRNA) assay,” and then incubated with DV (MOI = 5) as described in “DV infection to macrophages.” At 24 hours after infection, cytokine levels were determined by ELISA. Data are the mean ± SEM from 3 independent experiments. **P < .01; ***P < .001 (Student t tests). (D) Potassium efflux, cathepsin B activity, and syk signaling were essential for DV-triggered IL-1β/IL-18 production. LPS (5 ng/mL)–primed GM-Mφ were preincubated with DMSO, chemical inhibitors (APDC: 10, 30, 100μM; glibenclamide: 10, 30, 100μM, CA-074 Me: 1, 3, 10μM; Syk inhibitor: 1, 3, 10μM), followed by incubation with DV (MOI = 5) as described in “Blocking assay.” The levels of IL-1 β (left panel) and IL-18 (right panel) in the supernatants were detected by ELISA. Data are the mean ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001. (Student t tests). Gliben. indicates glibenclamide.
DV-induced IL-1β/IL-18 secretion is dependent on NLRP3 inflammasome activation in GM-Mφ. (A) Determination of cytokine mRNA levels by real-time PCR. GM-Mφ or M-Mφ were either incubated with DV directly (top panels) or primed with LPS (5 ng/mL) for 4 hours before incubation with DV (MOI = 5) for 2 hours at 37°C. Total RNAs from mock or DV-infected GM-Mφ and M-Mφ were isolated for reverse-transcription into cDNA at the indicated time point after infection. The expression levels was determined by real-time PCR and normalized with GAPDH. Fold change of gene expression in the y-axis was calculated based on the same gene expression in mock group in DV-infected macrophages (top panels), or based on the same gene expression in nontreated group (bottom panels). Data are the mean ± SEM from 3 independent experiments. *P < .05; **P < .01 (Student t tests). h.p.i indicates hours after infection. (B) Determination of NLR mRNAs by real-time PCR. The expression levels of NLRs after DV infection in both macrophages were determined by real-time PCR and normalized with GAPDH. Fold change of gene expression in the y-axis was calculated based on the same gene expression in mock group. Data are the mean ± SEM from 3 independent experiments. *P < .05; **P < .01 (Student t tests). (C) Knockdown NLRP3 by siRNA. LPS (5 ng/mL)–primed GM-Mφ were transfected with the nontargeting siRNA control (NT-i) or the NLRP3 siRNAs (NLRP3-i) as described in “RNA interference (siRNA) assay,” and then incubated with DV (MOI = 5) as described in “DV infection to macrophages.” At 24 hours after infection, cytokine levels were determined by ELISA. Data are the mean ± SEM from 3 independent experiments. **P < .01; ***P < .001 (Student t tests). (D) Potassium efflux, cathepsin B activity, and syk signaling were essential for DV-triggered IL-1β/IL-18 production. LPS (5 ng/mL)–primed GM-Mφ were preincubated with DMSO, chemical inhibitors (APDC: 10, 30, 100μM; glibenclamide: 10, 30, 100μM, CA-074 Me: 1, 3, 10μM; Syk inhibitor: 1, 3, 10μM), followed by incubation with DV (MOI = 5) as described in “Blocking assay.” The levels of IL-1 β (left panel) and IL-18 (right panel) in the supernatants were detected by ELISA. Data are the mean ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001. (Student t tests). Gliben. indicates glibenclamide.
In addition, NLRP3, but not NLRP1 and NPRC4, was up-regulated from 12 hours after DV infection in GM-Mφ (Figure 5B left panel). In contrast, DV failed to activate any of these NLRs in M-Mφ (Figure 5B right panel). Because NLRP3 expression is a critical indicator for NLRP3 inflammasome activation,35 NLRP3 siRNA was used to examine the role of NLRP3 inflammasome in IL-1β and IL-18 secretion. We found that siRNA suppressed both IL-1β and IL-18 secretion in DV-infected GM-Mφ (Figure 5C); thus, we conclude that DV can induce IL-1β and IL-18 secretion via activation of NLRP3 inflammasome. Several signals are essential for NLRP3 inflammasome activation (known as second signals for inflammasome activation), such as potassium efflux (required for extracellular ATP36 or other NLRP3 agonists),37-39 lysosomal cathepsin B release,40 ROS production,37 and Syk signaling.38,41 We found that glibenclamide (K+ channel inhibitor), CA-074 Me (cathepsin B inhibitor), and Syk inhibitor were able to suppress IL-1β and IL-18 secretion, whereas APDC (ROS inhibitor) had no effect in DV-infected GM-Mφ (Figure 5D). These observations suggest that DV-induced inflammasome activation involves K+ efflux, cathepsin B release from lysosomes, and is downstream to Syk activation. Thus, we conclude that DV may activate Syk-associating receptors to activate inflammasomes and induce the secretion of endogenous pyrogen (IL-1β) and IL-18.
DV-induced inflammasome activation is via CLEC5A
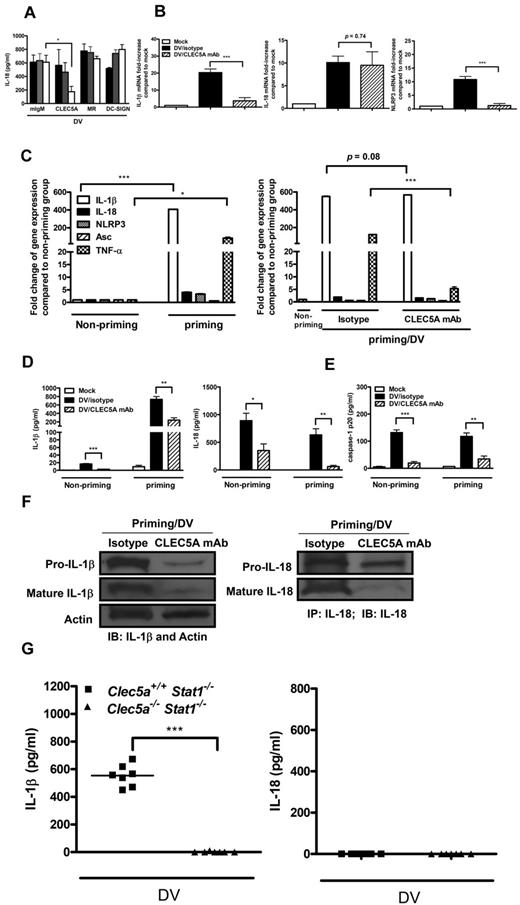
It has been shown that DV can bind and activate CLEC5A to induce the phosphorylation of DAP12,20 an ITAM-containing adaptor protein phosphorylated by activated Syk,42 as well as the release of proinflammatory cytokines. However, whether CLEC5A is linked to inflammasome activation has not been investigated yet. Thus, we examined whether the blockade of CLEC5A is able to inhibit DV-induced NLRP3 inflammasome activation in GM-Mφ. We found that antagonistic anti-CLEC5A mAb inhibited the secretion of IL-1β and IL-18 from GM-Mφ (supplemental Figure 6; Figure 6A). It is interesting to note that anti-CLEC5A mAb also inhibited the transcription of IL-1β and NLRP3, but not IL-18 in DV-infected GM-Mφ (Figure 6B), suggesting that CLEC5A may be critical in DV-induced NLRP3 inflammasome activation. We further investigated whether blockade of CLEC5A could still suppress NLRP3 inflammasome activation after LPS priming. We found that LPS successfully up-regulated the transcription of IL-1β, IL-18, NLRP3, and TNF-α in GM-Mφ (Figure 6C left panel). Even though blockade of CLEC5A was unable to suppress IL-1β mRNA transcription in GM-Mφ (Figure 6C right panel), the secretion of IL-1β and IL-18 (Figure 6D) as well as caspase-1 p20 in the supernatant (Figure 6E) were suppressed by anti-CLEC5A mAb. These observations suggest that anti-CLEC5A mAb-mediated effect is via inhibition of DV-induced NLRP3 inflammasome activation. The suppression of caspase-1 was in accordance with the decrease of mature IL-1β and IL-18 (Figure 6F). However, it was surprising to find that pro-IL-1β, but not pro-IL-18, was also suppressed by anti-CLEC5A mAb (Figure 6F). Therefore, we conclude that DV-induced NLRP3 inflammasome activation occurs via CLEC5A, and blockade of CLEC5A not only suppresses caspase-1-mediated maturation of IL-1β and IL-18, but also reduces pro-IL-1β. We further addressed the role of CLEC5A in DV-induced inflammasome activation using an established mice model for flaviviral infection.20,21 Whereas a high amount of IL-1β was observed in Clec5a+/+Stat1−/− mice, Clec5a−/−Stat1−/− mice did not produce any IL-1β after DV infection (Figure 6G left panel). This result provides direct evidence that CLEC5A is critical for DV-induced IL-1β production.
Inhibition of DV-activated NLRP3 inflammasomes by anti-CLEC5A mAb. (A) Blockade of CLEC5A inhibited the production of DV-induced IL-18. GM-Mφ were preincubated with anti-CLEC5A, anti-DC-SIGN, or anti-MR blocking antibodies for 1 hour, followed by incubation with DV (MOI = 5) for 2 hours at 37°C. At 48 hours after infection, culture supernatants were harvested to determine the levels of IL-18 by ELISA. Black, grey, and white bars indicated 0.1, 1, and 10 μg/well, respectively, as described in “Blocking assay.” *P < .05 (Student t test). (B) Anti-CLEC5A mAb inhibited the transcription of IL-1β and NLRP3 in DV-infected GM-Mφ. GM-Mφ were pre-incubated with CLEC5A mAb or isotype control for 1 hour, followed by incubation with DV (MOI = 5) as described in “Blocking assay.” Total RNAs from each group were isolated for reverse-transcription into cDNA at 24 hours after infection, and expression of cytokine and NLRP3 in each group was determined by real-time PCR. Gene expression was normalized by GAPDH. Fold change of gene expression in the y-axis was calculated based on the same gene expression in the mock group. ***P < .001 (Student t test). (C) Blockade of CLEC5A did not inhibit LPS-induced IL-1β mRNA transcription. Expression of cytokines, NLRP3, and Asc after priming for 4 hours in GM-Mφ was detected by real-time PCR and normalized by GAPDH (left panel). GM-Mφ were primed with LPS (5 ng/mL), followed by incubation with anti-CLEC5A mAb or isotype control before DV infection (MOI = 30) as described in “Blocking assay” (right panel). Total RNAs were harvested at 6 hours after infection for reverse-transcription into cDNA, and gene expression level was determined by real-time PCR and normalized by GAPDH. Fold change of gene expression in the y-axis was calculated based on the same gene expression in the nonpriming group. *P < .05; ***P < .001 (Student t tests). (D-E) Blockade of CLEC5A suppressed the secretion of IL-1β and IL-18 (D) and caspase-1 p20 (E) from LPS-primed GM-Mφ. LPS (5 ng/mL)–primed or nonprimed GM-Mφ were preincubated with CLEC5A mAb or isotype control, then incubated with DV (MOI = 30) as described in “Blocking assay.” At 24 hours after infection, supernatants were subjected to ELISA assay to determine cytokine levels and caspase-1 p20 amounts. Data in each figure are expressed as the mean ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001 (Student t tests). (F) Blockade of CLEC5A suppressed the expression and processing of pro-IL-1β and pro-IL-18. LPS (5 ng/mL)–primed GM-Mφ were preincubated with CLEC5A mAb or isotype control, then infected with DV (MOI = 30) as described in “Blocking assay.” At 24 hours after infection, cell lysates were harvested to determine the pro-form and mature form of IL-1β and IL-18. IL-1β was detected by Western blot. Actin was the internal control. IL-18 was pulled down from cell lysates by anti–IL-18 mAb before Western blot analysis. (G) CLEC5A controlled DV-induced IL-1β production. Clec5a+/+Stat1−/− and Clec5a−/−Stat1−/− mice (8 weeks) were inoculated intraperitoneally with 2 × 105 PFU/mice of DV2 (New Guinea C-N) as described previously.20 IL-1β (left panel) and IL-18 (right panel) in mice sera were measured by ELISA at day 7 after DV infection. Each dot represents an individual mouse; horizontal bars represent mean values for each group. ***P < .001 (Student t test).
Inhibition of DV-activated NLRP3 inflammasomes by anti-CLEC5A mAb. (A) Blockade of CLEC5A inhibited the production of DV-induced IL-18. GM-Mφ were preincubated with anti-CLEC5A, anti-DC-SIGN, or anti-MR blocking antibodies for 1 hour, followed by incubation with DV (MOI = 5) for 2 hours at 37°C. At 48 hours after infection, culture supernatants were harvested to determine the levels of IL-18 by ELISA. Black, grey, and white bars indicated 0.1, 1, and 10 μg/well, respectively, as described in “Blocking assay.” *P < .05 (Student t test). (B) Anti-CLEC5A mAb inhibited the transcription of IL-1β and NLRP3 in DV-infected GM-Mφ. GM-Mφ were pre-incubated with CLEC5A mAb or isotype control for 1 hour, followed by incubation with DV (MOI = 5) as described in “Blocking assay.” Total RNAs from each group were isolated for reverse-transcription into cDNA at 24 hours after infection, and expression of cytokine and NLRP3 in each group was determined by real-time PCR. Gene expression was normalized by GAPDH. Fold change of gene expression in the y-axis was calculated based on the same gene expression in the mock group. ***P < .001 (Student t test). (C) Blockade of CLEC5A did not inhibit LPS-induced IL-1β mRNA transcription. Expression of cytokines, NLRP3, and Asc after priming for 4 hours in GM-Mφ was detected by real-time PCR and normalized by GAPDH (left panel). GM-Mφ were primed with LPS (5 ng/mL), followed by incubation with anti-CLEC5A mAb or isotype control before DV infection (MOI = 30) as described in “Blocking assay” (right panel). Total RNAs were harvested at 6 hours after infection for reverse-transcription into cDNA, and gene expression level was determined by real-time PCR and normalized by GAPDH. Fold change of gene expression in the y-axis was calculated based on the same gene expression in the nonpriming group. *P < .05; ***P < .001 (Student t tests). (D-E) Blockade of CLEC5A suppressed the secretion of IL-1β and IL-18 (D) and caspase-1 p20 (E) from LPS-primed GM-Mφ. LPS (5 ng/mL)–primed or nonprimed GM-Mφ were preincubated with CLEC5A mAb or isotype control, then incubated with DV (MOI = 30) as described in “Blocking assay.” At 24 hours after infection, supernatants were subjected to ELISA assay to determine cytokine levels and caspase-1 p20 amounts. Data in each figure are expressed as the mean ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001 (Student t tests). (F) Blockade of CLEC5A suppressed the expression and processing of pro-IL-1β and pro-IL-18. LPS (5 ng/mL)–primed GM-Mφ were preincubated with CLEC5A mAb or isotype control, then infected with DV (MOI = 30) as described in “Blocking assay.” At 24 hours after infection, cell lysates were harvested to determine the pro-form and mature form of IL-1β and IL-18. IL-1β was detected by Western blot. Actin was the internal control. IL-18 was pulled down from cell lysates by anti–IL-18 mAb before Western blot analysis. (G) CLEC5A controlled DV-induced IL-1β production. Clec5a+/+Stat1−/− and Clec5a−/−Stat1−/− mice (8 weeks) were inoculated intraperitoneally with 2 × 105 PFU/mice of DV2 (New Guinea C-N) as described previously.20 IL-1β (left panel) and IL-18 (right panel) in mice sera were measured by ELISA at day 7 after DV infection. Each dot represents an individual mouse; horizontal bars represent mean values for each group. ***P < .001 (Student t test).
Discussion
The characteristic symptoms of dengue are sudden-onset fever, headache, muscle, and joint pains, and hepatosplenomegaly.43,44 The febrile phase involves high fever, often > 40°C (104°F), suggesting that DV may induce high amount of endogenous pyrogens (eg, IL-1β and TNF-α) to cause high fever in the victims. However, the underlying molecular mechanism has not been elucidated. Macrophage is regarded as the primary target of DV because DV can replicate and stimulate the secretion of inflammatory cytokines. In contrast to TNF-α, IL-1 is undetectable in DV-infected resting macrophages (M-Mφ; supplemental Figure 3) and cell lines (data not shown), and the source of IL-1β during DV infection was not elucidated before this study. Here, we clearly demonstrate that DV can induce massive IL-1β production from GM-Mφ via activation of NLRP3 inflammasome. To the best of our knowledge, this is the first report to show the role of NLRP3 in DV-induced IL-1 production, which would be important for high fever in dengue victims. It has been shown that IL-18, a type I cytokine with a strong proinflammatory effect, is associated with thrombocytopenia and increase of liver enzyme in DV patients.3 Thus, DV-induced thrombocytopenia may be mediated via the activation of NLRP3 to secrete IL-18.
The cytokine responsible for inflammation-induced splenomegaly is probably GM-CSF, as transgenic mice expressing GM-CSF under insulin promoter developed profound F4/80+ macrophage infiltration not only to pancreas, but also to spleen and liver.19 Because hepatosplenomegaly is frequently observed in flaviviral infections,43,44 and up-regulated GM-CSF and IL-1β serum levels correlate with disease severity,11 GM-Mφ is probably the dominant macrophage subset during flaviviral infection. This argument is supported by the observation that DV-infected M-Mφ did not produce IL-1β; thus, GM-Mφ seems to play a more important role than M-Mφ in the pathogenesis of DV infection. It will be interesting to investigate the role of GM-Mφ in other infectious diseases with hepatosplenomegaly in the future.
The importance of GM-CSF to regulate macrophage differentiation and response to pathogen invasion was reported in murine system previously.45,46 Rosas et al found that bone marrow–derived dendritic cells (cultured by GM-CSF) produced higher amounts of cytokines than BMMφ (cultured by M-CSF) in response to pathogenic fungi and its derivatives.45 While thioglycollate-elicted peritoneal Mφ did not respond to β-glucan, exogenous GM-CSF rendered cells producing high amount of cytokines to β-glucan, suggesting Mφ differentiation in vivo might be polarized by GM-CSF and had distinct response than primary Mφ in response to β-glucan stimulation.45 Goodridge et al reported that zymosan can induce higher amount of TNF-α in GM-CSF–derived BMDC than in BMMφ (cultured by M-CSF), and β-glucan can only induce TNF-α secretion from BMDCs, but not BMMφ. This observation suggests that GM-CSF can activate NF-κB to produce TNF-α induction on β-glucan stimulation,46 and GM-CSF is a potent cytokine to modulate cell functions in vivo. Moreover, it is interesting to find that β-glucan, which activates cell via dectin-1-CARD9 pathway, can only induce TNF-α secretion from BMDCs, but not BMMφ, even though both cell types express similar amount of dectin-1.46 Similarly, CLEC5A is expressed in both M-Mφ and GM-Mφ, but DV only induce the secretion of IL-1β and IL-18 via CLEC5A on GM-Mφ. Therefore, GM-CSF–triggered signaling may have synergistic effect with C-type lectin-mediated signaling. In addition, Pelegrin and Surprenant found that exogenous ATP activated NLRP3 inflammasome to produce IL-1β via induction of ROS in LPS/IFN-γ polarized murine BMMφ, whereas exogenous ATP inhibited ROS and was unable to activate NLRP3 inflammasome in IL-4–polarized murine BMMφ.47 This indicated that distinct BMMφ subset responds oppositely to ATP simulation. Therefore, the local environment of inflammatory sites has strong influences on macrophage differentiation and affect their response to same stimuli.
Previously, we have shown that CLEC5A is critical for DHF and DSS.20 In addition, Cheung et al show that pretreatment of concanavalin A causes the accumulation of CLEC5A+ myeloid cells in liver, and incubation of DV with the CLEC5A+ myeloid cells causes massive secretion of NO and TNF-α, whereas myeloid cells from CLEC5A−/− mice did not respond to DV infection under the same condition.48 Thus, the CLEC5A+ GM-Mφ subset may also play a critical role in the pathogenesis of DV infection. In contrast to IL-1β, however, IL-18 is undetectable in both Clec5a+/+Stat1−/− and Clec5a−/−Stat1−/− mice after DV infection. The underlying mechanism leading to the discrepancy between human and mouse in DV infection needs to be further characterized in the future. All of these observations suggest that blockade of CLEC5A seems to be able to alleviate clinical symptoms and prevent lethality in DV-infected victims via suppressing inflammasome activation and proinflammatory cytokine secretion.
Four inflammasomes (NLRP1, NLRP3, AIM2, and NLRC4) have been identified in macrophages. Among these 4 NLRs, NLRP3 inflammasome has obtained more attention because of its distinct function in antimicrobial responses, and sterile inflammatory responses.34 Recently, it has been shown that Syk kinase signaling couples to the NLRP3 inflammasome for antifungal defense.38 In this study, we found that DV could up-regulate the expression of NLRP3, whereas blockade of Syk-coupled C-type lectin CLEC5A was able to suppress NLRP3 inflammasome activation in DV-infected GM-Mφ. Thus, DV can activate NLPR3 inflammasome via CLEC5A.
Previous studies on DV-induced inflammatory cytokines rely on DV infection to M-Mφ20 or cell lines,49 which may not be able to reflect what happens during the inflammatory status in vivo. Compared with M-Mφ, GM-Mφ is more susceptible to DV infection and produces a higher amount of IL-1β, IL-18, TNF-α, (Figures 2D and 3A) as well as other mediators to increase vascular permeability as determined by in vitro assay (Figure 2E). This observation suggests that GM-CSF seems to be a potent factor to drive inflammasome activation on DV infection. It is interesting to note that DV cannot induce the secretion of IL-1β and IL-18 in M-Mφ (supplemental Figure 3), even though M-Mφ expresses higher amounts of innate immunity receptors/sensors (TLRs, and RIG-I) (Figure 1B-C) capable of transducing signal to up-regulate the transcription of pro-IL-1β and pro-IL-18. This indicates that differential responses between GM-Mφ and M-Mφ to DV infection may be attributed to the differential signaling machinery involved in the response to DV infection in both subsets. Compared with M-Mφ, abundant mitochondria were observed in GM-Mφ (supplemental Figure 1C). A recent study has demonstrated that mitochondria participate in a broad range of innate immune pathways, such as the regulation of antiviral signaling and antibacterial immunity, ROS production, autophagy, and cell death.50 Therefore, it will be interesting to investigate the role of GM-Mφ in host defense against bacterial, viral, and fungal infections in the future.
Our observations can explain why serum levels of IL-1β, GM-CSF,11 and IL-1810,11 correlate with disease severity in DV-infected patients. Higher levels of GM-CSF will skew macrophage differentiation into GM-Mφ, which produces IL-1β and IL-18 to increase body temperature and release soluble factors to increase vascular permeability on dengue infection. It would be interesting to understand whether the polymorphism of GM-CSF promoter is associated with increased amount of GM-CSF secretion, and correlates with disease severity after dengue infection in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Institute of Biomedical Sciences and National RNAi Core Facility, Academic Sinica, and BioLegend for their technique support and related antibody preparation as well as the Transgenic Mouse Model Core Facility of the National Core Facility Program for Biotechnology, National Science Council and the Gene Knockout Mouse Core Laboratory of National Taiwan University Center of Genomic Medicine for technical services.
This work was supported by the National Science Council (NSC 101-2325-B-010-006 and NSC 101-2321-B-010-003), Academia Sinica, and Thematic Research Project (AS-101-TP-B06-2), and in part by the Infection and Immunity Center, National Yang-Ming University, Taiwan (grant from Ministry of Education, Aim for the Top University Plan), Taipei Veterans General Hospital (V101E4-006, V101E4-007, and TVGH-NTUH VN-100-06), and the Molecular and Genetic Imaging Core/National Research Program for Genomic Medicine at National Yang-Ming University (NSC99-3112-B-010-015).
Authorship
Contribution: M.-F.W. designed, performed, and analyzed experiments and wrote the manuscript; S.-T.C., A.-H.Y., W.-W.L., Y.-L.L., N.-J.C., I.-S.T., and L.L. provided reagent and technique support; and S.-L.H. designed and analyzed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shie-Liang Hsieh, Institute of Clinical Medicine, National Yang-Ming University, 155, Section 2, Li-Nong Stt, Shih-Pai, Taipei, Taiwan 112; e-mail: slhsieh@ym.edu.tw.