Key Points
P-Rex1 is involved in E-selectin-mediated slow leukocyte rolling.
P-Rex1 mediates Mac-1-dependent intravascular crawling.
Abstract
Integrin activation is essential for the function of leukocytes. Impaired integrin activation on leukocytes is the hallmark of the leukocyte adhesion deficiency syndrome in humans, characterized by impaired leukocyte recruitment and recurrent infections. In inflammation, leukocytes collect different signals during the contact with the microvasculature, which activate signaling pathways leading to integrin activation and leukocyte recruitment. We report the role of P-Rex1, a Rac-specific guanine nucleotide exchanging factor, in integrin activation and leukocyte recruitment. We find that P-Rex1 is required for inducing selectin-mediated lymphocyte function-associated antigen-1 (LFA-1) extension that corresponds to intermediate affinity and induces slow leukocyte rolling, whereas P-Rex1 is not involved in the induction of the high-affinity conformation of LFA-1 obligatory for leukocyte arrest. Furthermore, we demonstrate that P-Rex1 is involved in Mac-1-dependent intravascular crawling. In vivo, both LFA-1-dependent slow rolling and Mac-1-dependent crawling are defective in P-Rex1−/− leukocytes, whereas chemokine-induced arrest and postadhesion strengthening remain intact in P-Rex1-deficient leukocytes. Rac1 is involved in E-selectin-mediated slow rolling and crawling. In vivo, in an ischemia-reperfusion–induced model of acute kidney injury, abolished selectin-mediated integrin activation contributed to decreased neutrophil recruitment and reduced kidney damage in P-Rex1-deficient mice. We conclude that P-Rex1 serves distinct functions in LFA-1 and Mac-1 activation.
Introduction
Integrins are adhesion molecules whose adhesiveness is regulated by conformational changes in their extracellular domain.1 The activation of leukocyte integrins represents a crucial event in inflammation.2 Playing a dual role in sensing and interacting with surrounding structures such as endothelium and extracellular matrix, integrins have proven essential for successful leukocyte attachment, extravasation, and intact cellular functioning.2 A disturbance of this process leads to an impaired inflammatory response.
Integrins are obligate heterodimers consisting of α- and β-subunits that each possess a short cytoplasmic tail and span the plasma membrane. Structural and functional evidence exists, especially for the lymphocyte function-associated antigen-1 (LFA-1), showing that at least three β2-integrin conformations exist: bent and extended with an open or closed headpiece.3 The resting integrin adopts a bent conformation that has a low ligand-blinding affinity.4 The extended conformation with a closed headpiece exhibits an intermediate-binding affinity, whereas the extended conformation with the open headpiece possesses a high affinity.3-5 The activation of different receptors induces different signaling cascades resulting in conformational changes and alterations of the binding affinity of integrins.3 In addition, ligand binding transduces signals from the extracellular domain to the cytoplasm in the classical outside-in direction.3
Leukocyte recruitment to the site of inflammation proceeds via complex interactions with the endothelium.2 Leukocytes tether to, roll along, firmly adhere to, and crawl on the endothelium before transmigrating out of the vasculature.2 Neutrophils roll along the vessel wall by interacting with E- and P-selectins, as well as with the LFA-1 ligand intercellular adhesion molecule-1 (ICAM-1).2,6 Selectin engagement induces a signaling pathway mediating partial LFA-1 activation with an intermediate ligand-binding affinity and slow leukocyte rolling.7-14 E-selectin binding to P-selectin glycoprotein ligand-1 (PSGL-1) induces the activation of a signaling pathway leading to the activation of the adhesion and degranulation-promoting adaptor protein (ADAP)8 and the Tec kinase Bruton tyrosine kinase (Btk).11,14 Btk and ADAP regulate 2 pathways.11 One pathway is phosphoinositide-3-kinase (PI3K) γ-dependent and the other pathway comprises phospholipase C (PLC) γ2, p38 mitogen-activated protein kinase, and Ras-related protein 1a (Rap1a).11,13 Both pathways regulate LFA-1-dependent slow rolling in vitro and in vivo.8,11,13 During rolling, neutrophils are exposed to chemokines presented on inflamed endothelium that bind to G-protein coupled receptors (GPCR) and fully activate LFA-1, which converts rolling to firm adhesion.15 However, the underlying signal transduction pathway following GPCR engagement is poorly understood.16
Crawling was first observed in vitro. Investigators reported that monocytes adhered, flattened, and subsequently formed pseudopods and crawled in an integrin-dependent manner to endothelial cell junctions where they subsequently transmigrated.17 The crawling process was necessary for emigration. Further studies demonstrated that Mac-1-dependent (αMβ2; macrophage-1 antigen) intraluminal crawling also appeared in vivo,17-19 and this allowed leukocytes to reach optimal emigration sites at endothelial junctions.17
The guanine exchange factor (GEF) P-Rex1 is 1 of approximately 20 GEFs that can activate the small GTPase Ras-related C3 botulinum toxin substrate (Rac) and has previously been identified to be the main Rac activator together with molecules of the Vav family.20,21 P-Rex1 usually translocates to the plasma membrane when activated by either phosphatidylinositol (3,4,5)-trisphosphate (PIP3), the product of phosphoinositide-3-kinase, or the βγ-subunit of G-proteins, and it may be inactivated by protein-kinase A-dependent phosphorylation.22,23 P-Rex1−/− mice had reduced neutrophil recruitment into the peritoneal cavity 4 hours after thioglycollate injection compared with wild-type (WT) mice.24 Also, P-Rex1-deficient mice showed impaired GPCR-induced generation of reactive oxygen species24 and P-Rex1 has recently been identified to mediate fMLP (N-formyl-methionyl-leucyl-phenylalanine)-induced neutrophil responses together with Vav family GEFs.20
The current study was designed to determine the role of P-Rex1 in integrin activation. Using functional ex vivo and in vivo assays, we find that P-Rex1 is involved in selectin-mediated LFA-1 activation and slow leukocyte rolling, but not in chemokine-induced arrest, thus demonstrating that P-Rex1 has distinct roles in LFA-1 activation. Furthermore, we demonstrate that P-Rex1 is required for Mac-1-dependent crawling. We directly assessed the roles of P-Rex1 in structural changes to LFA-1 and Mac-1 during activation using conformation-specific antibodies. Confirming our results in primary mouse neutrophils, we find that P-Rex1 is needed for selectin-mediated LFA-1 extension and slow leukocyte rolling, as well as Mac-1-dependent intravascular crawling.
Methods
Animals
The 8- to 12-week-old C57BL/6 mice (Janvier, Le Genest Saint Isle, France), LysM-GFP+ mice,25 P-Rex1−/− mice, and corresponding littermates24 were housed in the specified pathogen free (SPF) facility. The Animal Care and Use Committees of the University of Muenster (Muenster, Germany) approved all animal experiments.
Autoperfused flow chamber
Autoperfused flow chamber experiments were performed as previously described.7-9,11 Briefly, rectangular glass capillaries were coated with E-selectin (2.5 μg/mL) alone or in combination with ICAM-1 (2 μg/mL; R&D Systems, Wiesbaden-Nordenstadt, Germany) for 2 hours and then blocked for 1 hour using casein (Thermo Fisher Scientific, Bonn, Germany). To control the wall shear stress in the capillary, a PE-50 tubing (Becton Dickinson, Heidelberg, Germany) was connected to 1 side of the capillary. The other side of the chamber was connected to a PE-10 tubing (Becton Dickinson) and inserted into a mouse carotid artery. Leukocyte rolling was recorded for 1 minute using an SW40/0.75 objective and a digital camera (Sensicam QE, Cooke Corporation, Kelheim, Germany).
Adhesion flow chamber
Adhesion flow chamber experiments were carried out as previously described.10 Briefly, protein-G precoated glass capillaries were coated with E-selectin (6.6 µg/mL) and IgG1 (25 µg/mL) or KIM127 (25 µg/mL) for 1 hour and blocked with casein (Thermo Fisher Scientific, Bonn, Germany). In other experiments, capillaries were coated with P-Selectin (20 µg/mL), IL-8 (50 µg/mL, Peprotech, Rocky Hill, NJ), and IgG1 (5 µg/mL) or mAb24 (5 µg/mL, generous gift from N. Hogg). HL60-cells were resuspended in human plasma with a density of 5 × 106/mL living cells. The flow chamber was perfused with the cell suspension for 2 minutes and washed with phosphate-buffered saline (1 mM MgCl2/CaCl2) for 1 minute. In representative images, the number of cells per field of view was determined.
Intravital microscopy
Intravital microscopy of anesthetized mice was performed as described.7-9,11,13 To induce inflammation, mice received an intrascrotal injection of 500 ng tumor necrosis factor (TNF)-α or were superfused with macrophage-inflammatory protein 2 (MIP-2; R&D Systems, Wiesbaden-Nordenstadt, Germany) 2 hours before the exteriorization of the cremaster muscle. The cremaster muscle was prepared for intravital imaging as previously described.7,9,11,13 Intravital microscopy was carried out on an upright microscope (Axioskop; Carl Zeiss, Goettingen, Germany) with a 40× 0.75 NA saline immersion objective. Leukocyte rolling velocity and leukocyte arrest were determined by transillumination intravital microscopy, whereas leukocyte extravasation was investigated by near infrared reflected light oblique transillumination microscopy as previously described.11,13 Clustering of surface adhesion molecules (LFA-1) was performed as previously described.13 Recorded images were analyzed using ImageJ and AxioVision (Carl Zeiss) software. Emigrated cells were determined in an area reaching out 75 μm to each side of a vessel for a distance of 100 μm vessel length (representing 1.5 × 104 μm2 tissue area). The microcirculation was recorded using a digital camera (Sensicam QE, Cooke, Germany).
In some experiments, the rolling velocity of reconstituted leukocytes was measured as previously described.13,14 Briefly, bone marrow leukocytes from either WT or P-Rex1−/− mice were incubated with TAT-fusion mutants (1 μM, 37°C, 30 minutes) and PTx (200 ng/mL, 37°C, 2 hours), and then injected intravenously 30 minutes after the intrascrotal injection of TNF-α into LysM-GFP+ mice. Two hours after TNF-α application, the rolling velocity of the reconstituted leukocytes was measured in postcapillary venules of the cremaster muscle by intravital microscopy.
See “Methods” in supplemental data for further information on cell lines, constructs, confocal microscopy, the intravascular crawling assay, the in vitro crawling assay, the integrin activation assay, the selectin engagement assay, the renal ischemia-reperfusion injury, microscopy of the kidney, and statistics.
Results
P-Rex1 is required for E-selectin-mediated slow leukocyte rolling and Gαi-independent adhesion
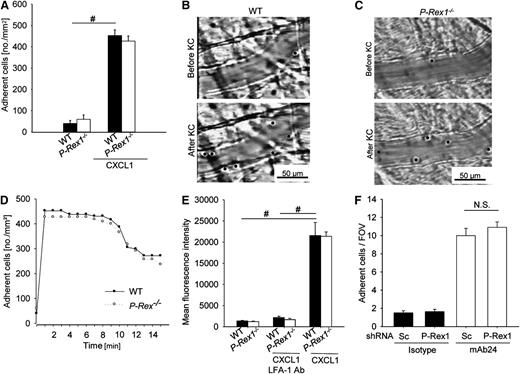
To examine the role of P-Rex1 in E-selectin-mediated slow rolling, the rolling velocity of P-Rex1−/− and WT mice was investigated using autoperfused flow chambers.7,9 The rolling velocity of WT neutrophils is significantly lower on E-selectin/ICAM-1 compared with E-selectin alone.7,11 Neutrophils from P-Rex1−/− mice showed a similar rolling velocity on E-selectin alone but revealed an elevated rolling velocity on E-selectin/ICAM-1 compared with WT neutrophils (Figure 1A). Since the reduction in rolling velocity of WT cells is LFA-1-dependent, we injected a blocking anti-LFA-1 antibody that elevated the rolling velocity even further to a level similar to E-selectin alone (Figure 1A). To support our flow chamber data, intravital microscopy of the postcapillary venules of the cremaster muscle was performed in P-Rex1−/− and WT mice. Leukocyte rolling velocity was analyzed after TNF-α pretreatment and blocking P-selectin and Gαi-signaling to assess E-selectin-mediated slow rolling.7 The mean rolling velocity of P-Rex1−/− leukocytes was significantly higher compared with WT control leukocytes (Figure 1B), but not as high as following the injection of a blocking anti-LFA-1 antibody, after which both WT and P-Rex1−/− leukocytes rolled at similarly high velocities (Figure 1B).
P-Rex 1 is required for E-selectin-mediated slow leukocyte rolling and Gαi-independent adhesion. (A) Carotid arteries of P-Rex1−/− mice (n = 3), P-Rex1−/− mice after injection of a blocking anti-LFA-1 antibody (n = 3) and WT mice (n = 3) were cannulated and connected to autoperfused flow chambers. Average rolling velocity of neutrophils on E-selectin (left) and E-selectin and ICAM-1 (right) is presented as means ± SEM. The wall shear stress in all flow chamber experiments was 5 to 6 dyn/cm2. (B) Cumulative histogram of rolling velocities of WT (n = 4) leukocytes before (▼) and after injection of a blocking anti-LFA-1 antibody (Δ) and corresponding values for P-Rex1−/− (n = 4) leukocytes (● and ○) in inflamed cremaster muscle venules treated with Pertussis Toxin (PTx) and a monoclonal blocking anti-P-selectin antibody. Inset data are means ± SEM. (C) Numbers of adherent cells per square millimeter in TNF-α inflamed cremaster venules after PTx pretreatment in WT (n = 4) and P-Rex1−/− (n = 4) mice and without PTx (n = 3 each). (D) Number of transmigrated cells per 1.5 × 104 μm2 in inflamed cremaster venules of WT (n = 4) and P-Rex1−/− (n = 4) mice with and without PTx (n = 3 each) as determined by near infrared reflected light oblique transillumination microscopy. (E) Representative images of inflamed cremasteric venules of WT (upper panel) and P-Rex1−/− (lower panel) mice as visualized using reflected light oblique transillumination microscopy. (F) Cumulative histogram depicting rolling velocities of leukocytes treated with either a cell-penetrating TAT peptide fused to a WT form (n = 3) of Rap1a (○) or to an dominant negative Rap1a (n = 3) with a S17N mutation (●) in inflamed cremaster venules of LysM GFP+ mice after pretreatment with PTx and injection of a blocking P-selectin antibody. Inset data are means ± SEM. (G) P-Rex1-knockdown HL-60 cells were generated using short hairpin RNA and knockdown efficiency of P-Rex1 was confirmed by western blot analysis. (H) HL-60 cells were analyzed using a flow chamber adhesion assay with E-selectin and either an antibody specific for the intermediate confirmation of LFA-1 (KIM127) or an isotype control. Adherent cells per field of view were counted and means ± SEM are displayed. (I) Clustering of LFA-1 during slow rolling of leukocytes in inflamed venules of the cremaster muscle in WT (n = 3) and P-Rex1−/− mice (n = 3). Bars are percentage of clustering cells ± SEM. (J) Representative images of WT (upper panel) and P-Rex1−/− (lower panel) rolling leukocytes stained with anti-LFA-1 antibody. #P < .05.
P-Rex 1 is required for E-selectin-mediated slow leukocyte rolling and Gαi-independent adhesion. (A) Carotid arteries of P-Rex1−/− mice (n = 3), P-Rex1−/− mice after injection of a blocking anti-LFA-1 antibody (n = 3) and WT mice (n = 3) were cannulated and connected to autoperfused flow chambers. Average rolling velocity of neutrophils on E-selectin (left) and E-selectin and ICAM-1 (right) is presented as means ± SEM. The wall shear stress in all flow chamber experiments was 5 to 6 dyn/cm2. (B) Cumulative histogram of rolling velocities of WT (n = 4) leukocytes before (▼) and after injection of a blocking anti-LFA-1 antibody (Δ) and corresponding values for P-Rex1−/− (n = 4) leukocytes (● and ○) in inflamed cremaster muscle venules treated with Pertussis Toxin (PTx) and a monoclonal blocking anti-P-selectin antibody. Inset data are means ± SEM. (C) Numbers of adherent cells per square millimeter in TNF-α inflamed cremaster venules after PTx pretreatment in WT (n = 4) and P-Rex1−/− (n = 4) mice and without PTx (n = 3 each). (D) Number of transmigrated cells per 1.5 × 104 μm2 in inflamed cremaster venules of WT (n = 4) and P-Rex1−/− (n = 4) mice with and without PTx (n = 3 each) as determined by near infrared reflected light oblique transillumination microscopy. (E) Representative images of inflamed cremasteric venules of WT (upper panel) and P-Rex1−/− (lower panel) mice as visualized using reflected light oblique transillumination microscopy. (F) Cumulative histogram depicting rolling velocities of leukocytes treated with either a cell-penetrating TAT peptide fused to a WT form (n = 3) of Rap1a (○) or to an dominant negative Rap1a (n = 3) with a S17N mutation (●) in inflamed cremaster venules of LysM GFP+ mice after pretreatment with PTx and injection of a blocking P-selectin antibody. Inset data are means ± SEM. (G) P-Rex1-knockdown HL-60 cells were generated using short hairpin RNA and knockdown efficiency of P-Rex1 was confirmed by western blot analysis. (H) HL-60 cells were analyzed using a flow chamber adhesion assay with E-selectin and either an antibody specific for the intermediate confirmation of LFA-1 (KIM127) or an isotype control. Adherent cells per field of view were counted and means ± SEM are displayed. (I) Clustering of LFA-1 during slow rolling of leukocytes in inflamed venules of the cremaster muscle in WT (n = 3) and P-Rex1−/− mice (n = 3). Bars are percentage of clustering cells ± SEM. (J) Representative images of WT (upper panel) and P-Rex1−/− (lower panel) rolling leukocytes stained with anti-LFA-1 antibody. #P < .05.
Intravascular firm adhesion of leukocytes in the cremasteric venules after TNF-α stimulation is mediated by E-selectin- and Gαi-signaling in an overlapping fashion.11 Even though P-Rex1−/− mice showed the same number of adherent and transmigrated leukocytes 2 hours after TNF-α application compared with WT mice, pretreatment with pertussis toxin (PTx) revealed significantly reduced numbers of adherent and transmigrated leukocytes in P-Rex1−/− mice (Figure 1C-E), suggesting that E-selectin-mediated integrin activation and leukocyte recruitment is diminished in P-Rex1−/− leukocytes. Using whole blood flow cytometry analysis, no difference in the expression levels of Mac-1, LFA-1, L-selectin or P-selectin glycoprotein ligand-1 (PSGL-1) was observed between WT and P-Rex1−/− neutrophils (data not shown).
P-Rex1 can be activated by PIP3, a product of phosphoinositide-3-kinase γ, and is a specific GEF for Rac.26 As selectin-mediated slow leukocyte rolling is only partially abrogated in P-Rex1-deficient mice, we investigated whether blocking Rap1a (the other pathway downstream of Btk) in the absence of P-Rex1 can completely abolish selectin-mediated slow leukocyte rolling. We blocked Rap1a by using a dominant-negative Rap1a TAT-fusion mutant13 in P-Rex1−/− leukocytes and investigated the rolling velocity of reconstituted leukocytes in postcapillary venules of the inflamed cremaster muscle (Figure 1F). Blocking Rap1a in P-Rex1−/− leukocytes increased the rolling velocity compared with P-Rex1−/− leukocytes treated with a WT Rap1a TAT-fusion peptide to velocities seen after blockade of LFA-1, suggesting that blocking Rap1a in P-Rex1−/− leukocytes completely abolishes E-selectin-mediated integrin activation and slow leukocyte rolling.
E-selectin engagement induces the activation of LFA-1, which is accompanied by the extended conformation of LFA-1.10 To directly scrutinize the role of P-Rex1 in selectin-mediated integrin activation, we used an immobilized reporter antibody assay to directly investigate the extended conformation of LFA-1.10 As reporter antibodies are only available for human cells, we stably knocked down P-Rex1 in the promyelocytic cell line HL-60 by transducing the cells with short hairpin RNA constructs against P-Rex1. Downregulation of P-Rex1 was confirmed by western blot analysis (Figure 1G). The immobilized reporter antibody assay revealed that the number of adherent cells per field of view was significantly reduced when the expression of P-Rex1 was down-regulated (Figure 1H).
Integrin clustering is another feature of integrin activation.8 To investigate the importance of P-Rex1 for LFA-1 clustering after E-selectin engagement, we performed intravital microscopy of the cremaster muscle. Clustering of surface adhesion molecules was determined as previously described,8,13 and the percentage of rolling leukocytes with clustered LFA-1 was calculated. In WT mice, LFA-1 clustered at the edge of rolling leukocytes. Furthermore, we demonstrated that >60% of WT leukocytes display LFA-1 clustering, whereas P-Rex1−/− leukocytes show a strong reduction of LFA-1 clustering (Figure 1I). Representative video micrographs of WT leukocytes and P-Rex1−/− leukocytes during E-selectin-mediated slow leukocyte rolling are shown in Figure 1J.
P-Rex 1 co-localizes with PIP3 at the plasma membrane leading to Rac1 activation and LFA-1 clustering
Previous reports have shown clustering of PIP3 at the plasma membrane together with Btk in a phosphoinositide-3-kinase-dependent manner.27,28 P-Rex1 has also been reported as being recruited to the plasma membrane when activated.23 To test whether P-Rex1 co-localizes with PIP3 at the plasma membrane after E-selectin stimulation, we used confocal microscopy of neutrophils. PIP3, PIP2 (phosphatidylinositol (4,5)-bisphosphate), and P-Rex1 distribution was uniform in unstimulated cells (Figure 2A-C). However, upon stimulation with E-selectin, PIP3 and to a lesser extent PIP2 clustered at the plasma membrane in WT and P-Rex1−/− neutrophils (Figure 2A-C). In stimulated WT neutrophils, P-Rex1 co-localized with PIP3, but not with PIP2, at the plasma membrane (Figure 2A-C). After stimulation with E-selectin, LFA-1 also clustered at the plasma membrane and co-localized with P-Rex1 and PIP3 in WT leukocytes (Figure 2D,F). In contrast to LFA-1, Mac-1 did not co-localize with the co-localized P-Rex1/PIP3 after stimulation with E-selectin (Figure 2E,F).
P-Rex1 co-localizes with PIP 3 at the plasma membrane leading to Rac1 activation and LFA-1 clustering. (A-B) Co-localization of P-Rex1 (red), PIP2 (green; A), and PIP3 (green; B) in WT (left) and P-Rex1−/− (right) neutrophils with and without E-selectin stimulation. Displayed are representative cells of 3 independent experiments. (C) Quantitative analysis of co-localization of PIP2 vs P-Rex1 and PIP3 vs P-Rex1, respectively. (D) Co-localization of P-Rex1 (red), PIP3 (green), and LFA-1 (purple) after E-selectin stimulation in WT and P-Rex1−/− neutrophils. Shown are representative images of 3 independent experiments. (E) Co-localization of P-Rex1 (red), PIP3 (green), and Mac-1 (purple) after E-selectin stimulation in WT and P-Rex1−/− neutrophils. Shown are representative images of 3 independent experiments. Scale bars indicate 10 μm. (F) Quantitative analysis of the co-localization of P-Rex1 and PIP3 with Mac-1 or LFA-1. (G) Representative western blots of Rac1 and Rac2 activity without stimulation and after stimulation with E-selectin in WT and P-Rex1−/− neutrophils (n = 3). (H) Densitometric analysis of Rac1 and Rac2 activity assays (n = 3) normalized to stimulated WT cells. Bars are adjusted relative density ± SEM. (I) Flow chamber of neutrophils preincubated with WT and DN Rac1 peptides on ICAM-1 and E-selectin/ICAM-1. (J) Corresponding flow chamber of neutrophils preincubated with WT and DN Rac2 peptides. #P < .05.
P-Rex1 co-localizes with PIP 3 at the plasma membrane leading to Rac1 activation and LFA-1 clustering. (A-B) Co-localization of P-Rex1 (red), PIP2 (green; A), and PIP3 (green; B) in WT (left) and P-Rex1−/− (right) neutrophils with and without E-selectin stimulation. Displayed are representative cells of 3 independent experiments. (C) Quantitative analysis of co-localization of PIP2 vs P-Rex1 and PIP3 vs P-Rex1, respectively. (D) Co-localization of P-Rex1 (red), PIP3 (green), and LFA-1 (purple) after E-selectin stimulation in WT and P-Rex1−/− neutrophils. Shown are representative images of 3 independent experiments. (E) Co-localization of P-Rex1 (red), PIP3 (green), and Mac-1 (purple) after E-selectin stimulation in WT and P-Rex1−/− neutrophils. Shown are representative images of 3 independent experiments. Scale bars indicate 10 μm. (F) Quantitative analysis of the co-localization of P-Rex1 and PIP3 with Mac-1 or LFA-1. (G) Representative western blots of Rac1 and Rac2 activity without stimulation and after stimulation with E-selectin in WT and P-Rex1−/− neutrophils (n = 3). (H) Densitometric analysis of Rac1 and Rac2 activity assays (n = 3) normalized to stimulated WT cells. Bars are adjusted relative density ± SEM. (I) Flow chamber of neutrophils preincubated with WT and DN Rac1 peptides on ICAM-1 and E-selectin/ICAM-1. (J) Corresponding flow chamber of neutrophils preincubated with WT and DN Rac2 peptides. #P < .05.
P-Rex1 has been described as a Rac-specific GEF.21,26 To test whether P-Rex1 in neutrophils is involved in Rac-activation after E-selectin stimulation, we performed a Rac activity assay. Rac1 and Rac2 get activated in E-selectin-stimulated WT neutrophils (Figure 2G,H). However, Rac2, but not Rac1, is activated in P-Rex1−/− neutrophils after stimulation with E-selectin (Figure 2G,H). By using blocking peptides, we demonstrated that only Rac1 is involved in E-selectin-mediated slow leukocyte rolling (Figure 2I,J).
Gαi-signaling is independent of the presence of P-Rex1
To investigate whether P-Rex1 is involved in chemokine-induced arrest, we conducted intravital microscopy of the cremaster muscle. WT mice and P-Rex1−/− mice had the same low number of adherent neutrophils under baseline conditions. Injection of the chemokine CXCL1, which binds to and activates CXCR2, induced firm arrest in WT and P-Rex1−/− mice (Figure 3A), suggesting that P-Rex1 is not involved in LFA-1 dependent chemokine-induced arrest. Representative video micrographs of WT mice and P-Rex1−/− mice before and after CXCL1 administration are shown in Figure 3B,C, respectively.
Gαi-signaling is independent of the presence of P-Rex1. (A) Adherent cells per square millimeter in cremaster venules before (left) and after (right) injection of 500 ng CXCL1 in WT (n = 4) and P-Rex1−/− (n = 5) mice ± SEM. (B-C) Representative images of venules before (upper panel) and after (lower panel) CXCL1 treatment in WT (B) and P-Rex1−/− (C) mice. (D) Numbers of adherent cells per square millimeter in untreated venules of the cremaster muscle of WT (n = 4) and P-Rex1−/− (n = 5) mice before and up to 15 minutes after CXCL1 injection. (E) ICAM-1 adhesion to CD45+, Ly-6B.2+ and Gr-1+ WT (n = 3) and P-Rex1−/− (n = 3) bone marrow cells without stimulation and after stimulation with CXCL1. Shown is mean fluorescence intensity of labeled ICAM-1 as determined by flow cytometry ± SEM. (F) HL-60 cells lines analyzed in a flow chamber adhesion assay coated with IL-8 and either an antibody specific for the open conformation of LFA-1 or isotype control. Depicted are mean adherent cells per field of view ± SEM. FOV, field of view; KC, keratinocyte-derived chemokine; Sc, scrambled. #P < .05.
Gαi-signaling is independent of the presence of P-Rex1. (A) Adherent cells per square millimeter in cremaster venules before (left) and after (right) injection of 500 ng CXCL1 in WT (n = 4) and P-Rex1−/− (n = 5) mice ± SEM. (B-C) Representative images of venules before (upper panel) and after (lower panel) CXCL1 treatment in WT (B) and P-Rex1−/− (C) mice. (D) Numbers of adherent cells per square millimeter in untreated venules of the cremaster muscle of WT (n = 4) and P-Rex1−/− (n = 5) mice before and up to 15 minutes after CXCL1 injection. (E) ICAM-1 adhesion to CD45+, Ly-6B.2+ and Gr-1+ WT (n = 3) and P-Rex1−/− (n = 3) bone marrow cells without stimulation and after stimulation with CXCL1. Shown is mean fluorescence intensity of labeled ICAM-1 as determined by flow cytometry ± SEM. (F) HL-60 cells lines analyzed in a flow chamber adhesion assay coated with IL-8 and either an antibody specific for the open conformation of LFA-1 or isotype control. Depicted are mean adherent cells per field of view ± SEM. FOV, field of view; KC, keratinocyte-derived chemokine; Sc, scrambled. #P < .05.
To test whether neutrophils from P-Rex1−/− mice have a postadhesion strengthening defect, adherent neutrophils were tracked after CXCL1 injection. Neutrophils from WT and P-Rex1−/− mice adhered rapidly to the endothelium after CXCL1 injection and remained attached for the next minutes (Figure 3D).
Various chemokines, such as CXCL1, are capable of inducing the high-affinity conformation of LFA-1 via GPCR. The binding of soluble ICAM-1 is commonly used to investigate the high-affinity state of LFA-1.29 To evaluate the role of P-Rex1 in chemokine-stimulated induction of the high-affinity conformation of LFA-1, neutrophils from WT and P-Rex1−/− mice were incubated with soluble murine ICAM-1/Fc. WT neutrophils showed minimal ICAM-1 binding under resting conditions but significant binding after activation with CXCL1, which was unaffected by pre-incubation with a blocking antibody against Mac-1 (data not shown) but completely blocked by pre-incubation with a blocking antibody against LFA-1 (Figure 3E). P-Rex1−/− neutrophils behaved similarly to WT neutrophils under baseline conditions and after CXCL1 stimulation (Figure 3E), demonstrating that P-Rex1 is not involved in chemokine-induced LFA-1-dependent arrest.
To directly test whether P-Rex1 is involved in GPCR-mediated integrin activation under flow conditions, we used the P-Rex1 knock-down HL-60 cell line in an immobilized reporter antibody assay.10 Here, we observed a similar number of adherent cells in both P-Rex1-knock-down and WT cells (Figure 3F), showing that P-Rex1 is not involved in GPCR-mediated LFA-1 activation.
P-Rex 1 is required for Mac-1 dependent intravascular crawling
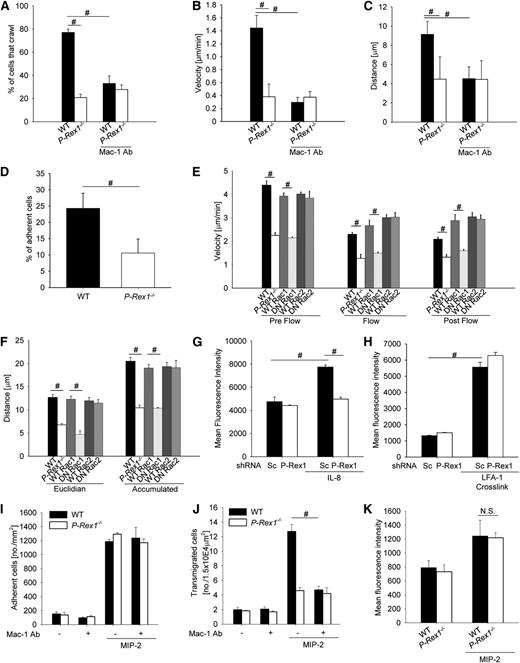
The β2-integrin Mac-1 has a critical role in intravascular crawling of leukocytes.17 To investigate the role of P-Rex1 in this step, we performed intravital microscopy of the cremaster muscle after stimulation with the chemokine MIP-2.17 The number of adherent leukocytes was similar between WT and P-Rex1−/− mice (data not shown). However, significantly fewer leukocytes crawled in P-Rex1−/− compared with WT mice (Figure 4A). Furthermore, P-Rex1−/− leukocytes had a reduced crawling velocity (Figure 4B) and distance (Figure 4C and supplemental Movie 1) compared with WT leukocytes (supplemental Movie 2). Blocking Mac-1 in WT mice significantly reduced the number of crawling cells (Figure 4A), crawling velocity (Figure 4B), and crawling distance (Figure 4C) to a level seen in P-Rex1−/− mice, whereas blocking Mac-1 in P-Rex1−/− mice did not further decrease the number of crawling leukocytes, crawling velocity, and crawling distance (Figure 4C).
P-Rex 1 is required for Mac-1 dependent intravascular crawling. (A-C) Intravascular crawling of Gr-1 labeled neutrophils in venules of the cremaster muscle during superfusion with MIP-2 (5 nM). Displayed are the percentage of adherent cells that crawled (A), mean crawling velocity of adherent cells (B), and mean distance crawled by adherent cells (C) in WT (n = 5) and P-Rex 1−/− (n = 5) and corresponding experiments after injection of a blocking anti-Mac-1 antibody (n = 3 each) mice ± SEM. (D-F) Isolated P-Rex1−/− and WT neutrophils were stimulated with MIP-2 and analyzed using a parallel plate flow chamber crawling assay coated with mouse serum. (D) Presented is the mean percentage of cells that remained adherent after applying flow of WT and P-Rex1−/− neutrophils (10 dyn/cm2, 2 minutes) ± SEM (n = 3). (E) Mean crawling velocity before (left), during (middle) and after (right) applying flow (2 dyn/cm2) ± SEM, (F) mean eucledian (left) and accumulated (right) crawled distance ± SEM of WT, P-Rex1−/− neutrophils and WT cells pretreated with WT or DN peptides for Rac1 or Rac2. (G) Mac-1 activation in HL-60 transfected with either scrambled or P-Rex1 short hairpin RNA after stimulation with IL-8 (n = 3). Bars are mean fluorescence intensity ± SEM as determined by FACS analysis using an antibody specific for the activated conformation of Mac-1. (H) Mac-1 activation after crosslinking of LFA-1 in HL-60 cells (n = 3). Displayed is mean fluorescence intensity ± SEM. (I) Adherent cells in the postcapillary venules of the cremaster muscle in WT and P-Rex1−/− mice with and without MIP-2 and with and without injection of a blocking anti-Mac-1 antibody. Displayed are adherent cells per mm2 ± SEM. (J) Corresponding analysis of transmigrated cells as cells per 1.5 × 104 μm2 ± SEM. (K) Mac-1 expression of blood neutrophils with and without MIP-2 stimulation as measured by flow cytometry. Presented as mean fluorescence intensity ± SEM. #P < .05.
P-Rex 1 is required for Mac-1 dependent intravascular crawling. (A-C) Intravascular crawling of Gr-1 labeled neutrophils in venules of the cremaster muscle during superfusion with MIP-2 (5 nM). Displayed are the percentage of adherent cells that crawled (A), mean crawling velocity of adherent cells (B), and mean distance crawled by adherent cells (C) in WT (n = 5) and P-Rex 1−/− (n = 5) and corresponding experiments after injection of a blocking anti-Mac-1 antibody (n = 3 each) mice ± SEM. (D-F) Isolated P-Rex1−/− and WT neutrophils were stimulated with MIP-2 and analyzed using a parallel plate flow chamber crawling assay coated with mouse serum. (D) Presented is the mean percentage of cells that remained adherent after applying flow of WT and P-Rex1−/− neutrophils (10 dyn/cm2, 2 minutes) ± SEM (n = 3). (E) Mean crawling velocity before (left), during (middle) and after (right) applying flow (2 dyn/cm2) ± SEM, (F) mean eucledian (left) and accumulated (right) crawled distance ± SEM of WT, P-Rex1−/− neutrophils and WT cells pretreated with WT or DN peptides for Rac1 or Rac2. (G) Mac-1 activation in HL-60 transfected with either scrambled or P-Rex1 short hairpin RNA after stimulation with IL-8 (n = 3). Bars are mean fluorescence intensity ± SEM as determined by FACS analysis using an antibody specific for the activated conformation of Mac-1. (H) Mac-1 activation after crosslinking of LFA-1 in HL-60 cells (n = 3). Displayed is mean fluorescence intensity ± SEM. (I) Adherent cells in the postcapillary venules of the cremaster muscle in WT and P-Rex1−/− mice with and without MIP-2 and with and without injection of a blocking anti-Mac-1 antibody. Displayed are adherent cells per mm2 ± SEM. (J) Corresponding analysis of transmigrated cells as cells per 1.5 × 104 μm2 ± SEM. (K) Mac-1 expression of blood neutrophils with and without MIP-2 stimulation as measured by flow cytometry. Presented as mean fluorescence intensity ± SEM. #P < .05.
Furthermore, we analyzed isolated P-Rex1−/− and WT neutrophils in an in vitro crawling assay using a parallel-plate flow chamber assay.30 Under nonflow conditions, WT and P-Rex1−/− neutrophils migrated randomly in all directions (data not shown). When shear stress (10 dyn/cm2) was applied, significantly fewer P-Rex1−/− neutrophils stayed adherent compared with WT neutrophils (Figure 4D). The crawling velocity of P-Rex1−/− neutrophils was significantly reduced under nonflow and flow conditions compared with WT neutrophils (Figure 4E). Furthermore, P-Rex1−/− neutrophils had a significantly reduced euclidean and accumulated distance compared with WT neutrophils (Figure 4F). By using blocking peptides, we demonstrated that only Rac1, but not Rac2, reduced crawling in vitro (Figure 4E,F).
To show that chemokine-induced Mac-1 activation is defective in the absence of P-Rex1, we assessed the binding of a reporter antibody that only detects the activated form of Mac-131 using flow cytometry. After stimulation with IL-8, the downregulation of P-Rex1 in HL-60 cells significantly abolished Mac-1 activation compared with control HL-60 cells (Figure 4G).
To examine if Mac-1 activation after outside-in signaling is impaired in P-Rex1-knock-down HL-60 cells, we investigated the binding of the reporter antibody against the activated form of Mac-131 after crosslinking of LFA-1 using antibodies. Crosslinking β2-integrins is known to cause Mac-1 activation.32 HL-60 cells with down-regulated P-Rex1 did not exhibit impaired Mac-1 activation after crosslinking compared with control HL-60 cells (Figure 4H), suggesting that outside-in signaling is intact in P-Rex1-knock-down HL-60 cells.
By using intravital microscopy, we investigated neutrophil adhesion and emigration in WT and P-Rex1−/− mice in the presence and absence of a blocking anti-Mac-1-antibody (Figure 4I,J). WT and P-Rex1−/− mice showed the same number of adherent neutrophils (Figure 4I), but the number of emigrated neutrophils in P-Rex1−/− was significantly lower compared with WT mice (Figure 4J). Blocking Mac-1 did not influence the number of adherent neutrophils in WT mice and P-Rex1−/− mice (Figure 4I), but reduced the number of emigrated neutrophils in WT mice (Figure 4J). However, the anti-Mac-1 antibody did not affect the number of emigrated neutrophils in P-Rex1-deficient mice (Figure 4J), suggesting that Mac-1-regulated neutrophil emigration is defective in P-Rex1−/− mice. Furthermore, we investigated the surface expression of Mac-1 on WT and P-Rex1−/− neutrophils before and after MIP-2 superfusion by flow cytometry. WT and P-Rex1−/− neutrophils had the same surface expression of Mac-1 before and after stimulation (Figure 4K).
Mice deficient for P-Rex1 are protected from renal ischemia-reperfusion injury
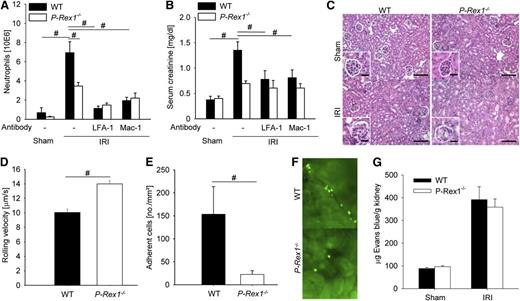
E-selectin signaling has been previously shown to be crucially important for the development of acute kidney injury (AKI) after ischemia-reperfusion.8 To test the physiological importance of P-Rex1 for neutrophil recruitment and consecutive organ failure after ischemia-reperfusion injury (IRI), P-Rex1−/− and WT mice were subjected to 32 minutes clamping of the kidney pedicle. After reperfusion, WT mice showed an elevated number of neutrophils in the kidney compared with sham animals after 24 hours (Figure 5A). Concomitant with the neutrophil influx, WT mice that had undergone 32 minutes of ischemia showed an increase in creatinine concentration 24 hours after reperfusion (Figure 5B). Blockade of LFA-1 or Mac-1 decreased neutrophil recruitment and preserved organ function (Figure 5A-B). Blocking both β2-integrins did not further decrease neutrophil recruitment into the kidney after IRI (data not shown). The IRI produced great morphological damages in the kidneys from WT mice, including massive tubular edema, cell recruitment, and loss of tubular epithelial cells (Figure 5C). P-Rex1−/− mice revealed medulla hyperemia but reduced cell recruitment and cellular destruction (Figure 5C).
Mice deficient for P-Rex1 are protected from renal ischemia-reperfusion injury. (A) Mean numbers of neutrophils per kidney 24 hours after ischemia-reperfusion injury or sham operation as determined by flow cytometry analysis ± SEM in WT mice (n = 5), P-Rex1−/− mice (n = 4), and WT mice pretreated with a blocking antibody against LFA-1 (n = 3) or Mac-1 (n = 3). (B) Serum creatinine levels in the blood of WT mice (n = 5), P-Rex1−/− mice (n = 4), and WT mice pretreated with a blocking antibody against LFA-1 (n = 3) or Mac-1 (n = 3) subjected to ischemia-reperfusion injury and sham operation (n = 3 each) 24 hours after the intervention ± SEM. (C) Representative hematoxylin and eosin stainings of kidney outer medulla from WT mice and P-Rex1−/− mice were assessed 24 hours after sham operation or renal IRI. Bar represents 50 μm. (D) Rolling velocities of labeled WT (n = 3) and P-Rex1−/− (n = 3) neutrophils 4 hours after ischemia reperfusion in cortical venules of the kidney. Bars are means ± SEM. (E) Mean numbers of labeled adherent cells per square millimeter in cortical kidney venules 4 hours after ischemia-reperfusion injury in WT mice injected with either labeled WT (n = 3) or P-Rex1−/− (n = 3) bone marrow ± SEM. (F) Representative image of renal cortical venules 4 hours after ischemia-reperfusion injury in mice injected with labeled WT (above) or P-Rex1−/− (below) bone marrow. (G) Permeability of kidney vessels as measured by quantification of extravasated Evans blue in WT mice and P-Rex1−/− mice after sham operation or ischemia-reperfusion injury ± SEM. #P < .05.
Mice deficient for P-Rex1 are protected from renal ischemia-reperfusion injury. (A) Mean numbers of neutrophils per kidney 24 hours after ischemia-reperfusion injury or sham operation as determined by flow cytometry analysis ± SEM in WT mice (n = 5), P-Rex1−/− mice (n = 4), and WT mice pretreated with a blocking antibody against LFA-1 (n = 3) or Mac-1 (n = 3). (B) Serum creatinine levels in the blood of WT mice (n = 5), P-Rex1−/− mice (n = 4), and WT mice pretreated with a blocking antibody against LFA-1 (n = 3) or Mac-1 (n = 3) subjected to ischemia-reperfusion injury and sham operation (n = 3 each) 24 hours after the intervention ± SEM. (C) Representative hematoxylin and eosin stainings of kidney outer medulla from WT mice and P-Rex1−/− mice were assessed 24 hours after sham operation or renal IRI. Bar represents 50 μm. (D) Rolling velocities of labeled WT (n = 3) and P-Rex1−/− (n = 3) neutrophils 4 hours after ischemia reperfusion in cortical venules of the kidney. Bars are means ± SEM. (E) Mean numbers of labeled adherent cells per square millimeter in cortical kidney venules 4 hours after ischemia-reperfusion injury in WT mice injected with either labeled WT (n = 3) or P-Rex1−/− (n = 3) bone marrow ± SEM. (F) Representative image of renal cortical venules 4 hours after ischemia-reperfusion injury in mice injected with labeled WT (above) or P-Rex1−/− (below) bone marrow. (G) Permeability of kidney vessels as measured by quantification of extravasated Evans blue in WT mice and P-Rex1−/− mice after sham operation or ischemia-reperfusion injury ± SEM. #P < .05.
To directly visualize leukocyte rolling and adhesion in the kidney and investigate the role of P-Rex1 in these steps, we performed intravital microscopy of the kidney after inducing ischemia-reperfusion–induced AKI. P-Rex1−/− leukocytes had an elevated rolling velocity and reduced leukocyte adhesion compared with WT leukocytes (Figure 5D-E). Shear rates and diameters were similar between different groups, excluding a hemodynamic contribution to reduced leukocyte rolling velocity and adhesion (data not shown). Representative video micrographs of kidneys from WT mice reconstituted with WT or P-Rex1−/− leukocytes after IRI are shown in Figure 5F. These data demonstrate that the protection from renal IRI seen in P-Rex1−/− mice is at least partly caused by decreased E-selectin-mediated slow leukocyte rolling and adhesion.
Furthermore, we measured vascular permeability in the kidney of WT and P-Rex1−/− mice under baseline conditions and after ischemia reperfusion injury. WT mice and P-Rex1−/− mice had the same vascular permeability under baseline conditions (Figure 5G). The vascular permeability increased to a same extent in WT mice and P-Rex1−/− mice after renal ischemia reperfusion injury (Figure 5G).
Discussion
Integrin activation is crucial for numerous leukocyte responses, including slow rolling, adhesion, crawling, migration, and phagocytosis. During leukocyte recruitment, leukocytes collect different proinflammatory mediators that may regulate integrin activation. However, the molecular mechanisms underlying integrin activation and neutrophil recruitment to sites of inflammation have proved elusive. Here, we show that the guanine exchange factor P-Rex1 is involved in E-selectin-mediated LFA-1 activation and slow leukocyte rolling, and is required for Mac-1-dependent intravascular crawling of neutrophils. Elimination of P-Rex1 improved renal function and reduced neutrophil recruitment in a mouse model of ischemia-reperfusion-induced AKI.
Activation of phosphoinositide-3-kinase γ has been previously shown to transduce E-selectin-mediated integrin activation downstream of Btk11 and leads to the production of PIP3, a second messenger that may activate P-Rex1.23 P-Rex1-deficient mice showed the same phenotype in E-selectin-mediated slow leukocyte rolling as seen in phosphoinositide-3-kinase γ-deficient mice.11 Although the rolling velocity of P-Rex1−/− neutrophils was only partially elevated on E-selectin/ICAM-1, this resulted in a reduced leukocyte adhesion in vivo in the absence of Gαi-dependent signaling. This is the first study that suggests a potential role of Rac1 in E-selectin-mediated slow leukocyte rolling.
Since phosphoinositide-3-kinase has been reported to be involved in GPCR-mediated firm arrest and postadhesion strengthening,33 we expected that P-Rex1−/− leukocytes exhibit impaired integrin activation to the high-affinity conformation after chemokine stimulation. However, our data revealed no phenotype in GPCR-mediated integrin activation and postadhesion strengthening after stimulation with CXCL1. This observation is in line with recent reports showing an overlapping redundancy of P-Rex1 and Vav1 for GPCR-mediated leukocyte adhesion and chemotaxis after stimulation with N-formyl-methionyl-leucyl-phenylalanine (fMLP).20 One explanation for the specific function of P-Rex1 could be that in some signaling pathways only some specific GEFs are activated. Another explanation could be the compartmentalization of GTPase regulators. It has been shown that different GTPase regulators are localized in different compartments and that the specific distribution of the different GTPase regulators is important for cell shape changes and movements.34 In contrast to these findings, elimination of CalDAG-GEFI, a GEF required for the activation of Rap GTPases, abolishes chemokine-induced arrest of primary neutrophils.13 However, our data clearly demonstrate that P-Rex1 is involved in Mac-1 activation after stimulation of HL-60 cells with IL-8, suggesting that different signaling pathways exist for the activation of different β2-integrins.
GPCR- and E-selectin-triggered signaling pathways regulate integrin affinity and avidity.8,35 Blocking E-selectin-mediated signaling by using P-Rex1-deficient neutrophils abolished integrin activation (affinity and avidity). P-Rex1 has been shown to translocate to the plasma membrane when activated.23 Our finding that P-Rex1 translocates to the plasma membrane and clusters together with PIP3 when stimulated with E-selectin confirms the published observation.23 LFA-1 clusters at the same site of the plasma membrane as P-Rex1 and PIP3. As the upregulation of the integrin activity is disturbed in P-Rex1-knock-down HL-60 cells, these data suggest that P-Rex1 is involved in the regulation of integrin affinity and avidity after E-selectin stimulation, but not after chemokine stimulation.
It is assumed that the 2 β2-integrins (ie, LFA-1 and Mac-1) act in a sequential fashion during leukocyte recruitment. After LFA-1-dependent adhesion, the binding of ligands to LFA-1 transmits signals into the cell (outside-in signaling) and induces cytoskeletal rearrangements as well as Mac-1 activation probably leading to crawling.30 Elimination of Vav1, which is involved in integrin-mediated outside-in signaling,36 disturbed the complex interplay between LFA-1 and Mac-1 and consequently altered intravascular crawling and reduced migration of neutrophils out of the inflamed microvasculature.30 In contrast to Vav1−/− neutrophils, we demonstrated that P-Rex1 is not involved in integrin-mediated outside-in signaling by measuring the activation status of Mac-1 after crosslinking LFA-1 in control HL-60 cells and P-Rex1-knock-down HL-60 cells. However, our reporter binding assay demonstrates that P-Rex1 is involved in chemokine-induced Mac-1 activation, unlike chemokine-induced LFA-1 activation. Elimination of P-Rex1 diminishes Rac activation.26 It has been shown that Rac1 and Rac2 both regulate distinct and overlapping cell functions in hematopoietic cells.37-39 However, only Rac1 is involved in E-selectin-mediated slow rolling and crawling.
One important hallmark of AKI is a strong recruitment of neutrophils into kidneys.40,41 Blocking E-selectin has a beneficial effect on renal42 and myocardial43 hypoxia-induced inflammation. In ischemic tissues, activated transcription factor HIF elicits profound changes of the vascular microenvironment, including an increase of recruitment, activation, and invasiveness of myeloid cells.44 It has been shown that neutrophils are recruited into the inflamed kidneys and account for tissue injury and impairment of kidney function after renal IRI.40,41 As the E-selectin-mediated signaling pathway is the major pathway for leukocyte recruitment into the inflamed kidney,8 blocking this pathway abolishes the number of neutrophils in the injured kidneys and consequently attenuates the severity of the AKI. This study demonstrates an important role of P-Rex1 in E-selectin-mediated signaling, integrin activation, and adhesion, and consequently in ischemia-reperfusion-induced organ failure. By using intravital microscopy and in vitro assays, we demonstrated that P-Rex1 is involved in LFA-1-dependent slow leukocyte rolling and Mac-1-dependent crawling. However, based on the facts that blocking both β2-integrins did not further decreased neutrophil recruitment into the kidney compared with blocking only 1 β2-integrin and the impossibility of visualizing leukocyte crawling in the microcirculation of the kidney, we cannot conclude which of the defective signaling pathways causes the reduced neutrophil recruitment into the kidney of P-Rex1−/− mice.
These data demonstrate the physiological relevance of the E-selectin-mediated signaling pathway in vivo. The new mechanistic insights we provide in this study suggest that P-Rex1 may be a promising therapeutic target for anti-inflammatory therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Klaus Ley for critical revision of the manuscript, Nancy Hogg for providing the mAb24 antibody, and Michael Glogauer for providing the Rac2 constructs.
This study was supported by grants from the German Research Foundation (AZ 428/3-1, AZ 428/6-1, SFB 1009/A5 to A.Z. and HE-6810/1-1 to J.H.) and the Interdisciplinary Center for Clinical Research (IZKF) (SEED 01/12 to J.R.).
Authorship
Contribution: J.M.H. performed experiments and helped analyzing the data. J.R. performed experiments and helped analyzing the data. H.B. performed experiments. H.W. provided knock-out animals and revised the manuscript. A.Z. designed the study, analyzed the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Zarbock, University of Münster, Department of Anaesthesiology, Intensive Care and Pain Medicine, Albert-Schweitzer-Campus 1, Building A1, 48149 Münster, Germany; e-mail: zarbock@uni-muenster.de.
References
Author notes
J.M.H. and J.R. contributed equally to this study.