To the editor:
Lymphocytic variant hypereosinophilic syndrome (LHES) is a rare disease in which cytokine production by T cells drives blood and tissue eosinophilia.1,2 We report a case of LHES in a patient with chronic active Epstein-Barr virus (CAEBV) infection and an EBV-infected T-cell clone producing eosinophilopoietic cytokines. All methods can be found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
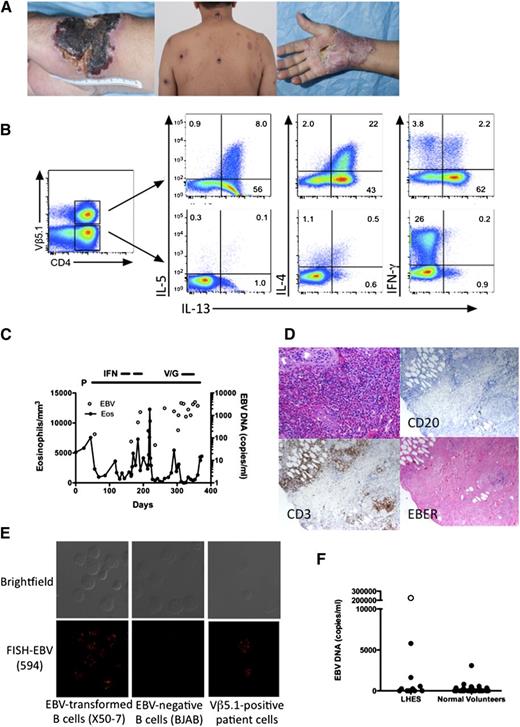
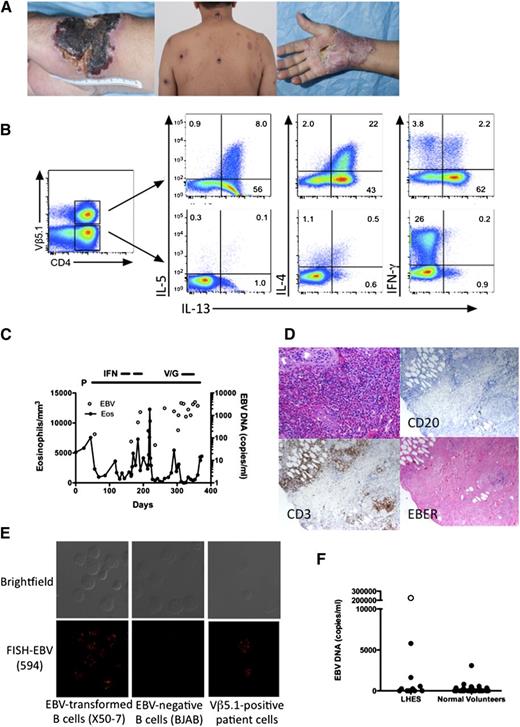
A 64-year-old man presented with a 2-year history of pruritic plaques on the extremities, trunk, and face that ulcerated and formed eschars with residual scarring (Figure 1A left and center panels). Laboratory testing revealed eosinophilia (5111/mm3), elevated serum immunoglobulin E (IgE) (13 995 ng/mL), and a clonal T-cell receptor pattern (supplemental Figure 1). Flow cytometry identified clonal Vβ5.1-positive CD3+CD4+CD25+ T lymphocytes representing 22% of peripheral blood lymphocytes (supplemental Figure 2). Intracellular cytokine staining confirmed a markedly increased percentage of Vβ5.1-positive CD4+ T cells producing Th2 cytokines (interleukin-4 [IL-4], IL-5, IL-13) (Figure 1B). Bone marrow biopsy was normocellular with eosinophilia and no lymphocytosis. Cytogenetic studies and testing for FIP1L1/PDGFRA were negative.
Clinical and laboratory manifestations of EBV-driven LHES. (A) Skin lesions before (left and center panels) and during (right panel) prednisone therapy. (B) Intracellular production of IL-4, IL-5, IL-13, and IFN-γ by viable CD3+CD4+ Vβ5.1-positive (top panels) and -negative (bottom panels) T cells. (C) Absolute eosinophil count and EBV DNA in the blood as a function of time. P, prednisone; IFN, interferon-α; V/G, vorinostat/ganciclovir. (D) Paraffin-embedded sections of a skin biopsy stained with hematoxylin and eosin (top left panel) showing dense eosinophilic infiltrate in the upper dermis, antibody to CD3 (bottom left panel), antibody to CD20 (top right panel) and in situ hybridization for EBER (bottom right panel). Magnification, ×400. (E) EBV DNA localization by fluorescence in situ hybridization showing EBV DNA in the nucleus of EBV-positive transformed B cells and in the patient’s Vβ5.1-positive T cells, but not in EBV-negative B cells. (F) Quantitative EBV DNA PCR in peripheral blood from normal volunteers and patients with LHES. Symbols represent individual subject data. ○, The value for the subject with EBV-driven LHES.
Clinical and laboratory manifestations of EBV-driven LHES. (A) Skin lesions before (left and center panels) and during (right panel) prednisone therapy. (B) Intracellular production of IL-4, IL-5, IL-13, and IFN-γ by viable CD3+CD4+ Vβ5.1-positive (top panels) and -negative (bottom panels) T cells. (C) Absolute eosinophil count and EBV DNA in the blood as a function of time. P, prednisone; IFN, interferon-α; V/G, vorinostat/ganciclovir. (D) Paraffin-embedded sections of a skin biopsy stained with hematoxylin and eosin (top left panel) showing dense eosinophilic infiltrate in the upper dermis, antibody to CD3 (bottom left panel), antibody to CD20 (top right panel) and in situ hybridization for EBER (bottom right panel). Magnification, ×400. (E) EBV DNA localization by fluorescence in situ hybridization showing EBV DNA in the nucleus of EBV-positive transformed B cells and in the patient’s Vβ5.1-positive T cells, but not in EBV-negative B cells. (F) Quantitative EBV DNA PCR in peripheral blood from normal volunteers and patients with LHES. Symbols represent individual subject data. ○, The value for the subject with EBV-driven LHES.
Quantitative EBV DNA polymerase chain reaction (PCR) showed 147 000 copies per mL of blood (normal <200) with the highest levels in T cells (79 891 copies per million T cells, 15 675 copies per million B cells, and 38 376 copies per million non-T, non-B cells). EBV DNA was localized in 70% of Vβ5.1-positive T cells at 8 to 25 copies per cell (Figure 1E), and DNA from sorted Vβ5.1-positive T cells showed a single band on Southern blot using a probe to the EBV terminal repeats, indicating that the virus infecting the Vβ5.1-positive T cells was clonal (supplemental Figure 3). Skin biopsy prior to therapy showed eosinophilic infiltration and EBV-encoded RNA (EBER) staining consistent with EBV RNA in T cells (Figure 1D). Scattered EBV-positive cells were also noted in the bone marrow biopsy (supplemental Figure 4).
Prednisone resulted in improvement in skin lesions (Figure 1A right panel) and eosinophilia, but had no effect on EBV DNA levels (Figure 1C). Biopsy of a papular lesion showed a clonal EBV-positive T-cell infiltrate consistent with hydroa vacciniforme. Interferon-α(IFN-α), lenalidomide, and vorinostat and valganciclovir were ineffective in reducing the EBV viral load, and the patient continues on prednisone 10 mg daily.
EBV DNA PCR was performed using peripheral blood from 15 subjects with LHES and 31 normal volunteers (Figure 1F). Slightly elevated EBV DNA levels (3100, 1650, and 5800 copies per mL) were detected in 1 normal subject and 2 patients with LHES, 1 of whom had pruritic skin lesions. Retesting of blood from this patient was again positive at 11 800 copies per mL, but EBER staining of skin and bone marrow biopsies was negative.
Although our patient meets criteria for CAEBV disease based on the chronic presence of high levels of EBV in the blood, skin, and bone marrow,3,4 his clinical presentation was atypical and more consistent with LHES with peripheral and tissue eosinophilia caused by an EBV-infected Vβ5.1 T-cell clone producing Th2 cytokines. Although EBV is not a common cause of LHES, screening for EBV infection should be considered in patients presenting with eosinophilia and a T-cell clone.
The online version of this article contains a data supplement.
Authorship
The online version of this article contains a data supplement.
Acknowledgments: The authors acknowledge the Warren Magnusson Clinical Center support staff, in general, and Eunice Fox, Lauren Pauls, and Amara Pabon, in particular, for their participation in the care of the study subject.
This work was supported in whole or in part by the intramural research programs of the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the Clinical Center at the National Institutes of Health and with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN2610080001E.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Contribution: A.D.K. and J.I.C. designed the research, collected, analyzed, and interpreted the data, performed statistical analysis, and wrote the manuscript; C.P. designed research, analyzed and interpreted the data, and helped write the manuscript; R.M., E.W.C., M.S.-S., S.P., I.M., M.R., L.X., and G.A.F. collected and interpreted clinical data and helped write the manuscript; K.C.D., T.K., C.S., A.N.S., and Y.Y. performed research and analyzed data; K.D. helped design the research; and N.H.-T. and S.-P.T. collected data and coordinated the clinical research studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amy D. Klion, National Institutes of Health, Building 4, Room B1-28, 4 Memorial Dr, Bethesda, MD 20892; e-mail: aklion@nih.gov.