Megakaryocytes in the hematopoietic niche extrude thin proplatelet processes or “arms” that pass through blood vessel walls, enter their lumen, and release platelets into the circulation. Precisely how proplatelet arms work their way into blood vessels is poorly understood. In this issue of Blood, Schachtner and colleagues1 show that megakaryocytes respond to extracellular matrices (ECMs) by assembling podosomes, specialized plasma membrane structures that promote cell motility and invasiveness, suggesting their potential role in proplatelet arm extension across basement membranes.
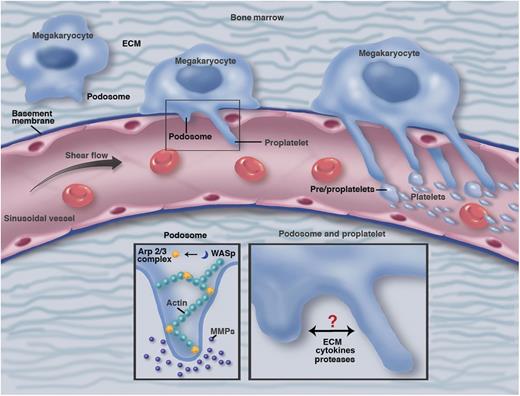
A hypothetical model showing bone marrow megakaryocytes transitioning (left to right) from the endosteal to vascular niche generating podosome structures in response to ECM and vascular sinusoidal basement membrane. When megakaryocytes breach the basement membrane, they extend proplatelet arms through the sinusoidal endothelium into the bloodstream. In response to ECM cues, megakaryocytes generate classical podosome structures containing actin cores induced by Arp2/3 and WASp (bottom left). These podosome structures degrade ECM and basement membrane via secreted and/or transmembrane MMPs. Putative crosstalk between these protease releasing structures and proplatelet arms extending into vessels (bottom right) remains to be determined. Professional illustration by Marie Dauenheimer.
A hypothetical model showing bone marrow megakaryocytes transitioning (left to right) from the endosteal to vascular niche generating podosome structures in response to ECM and vascular sinusoidal basement membrane. When megakaryocytes breach the basement membrane, they extend proplatelet arms through the sinusoidal endothelium into the bloodstream. In response to ECM cues, megakaryocytes generate classical podosome structures containing actin cores induced by Arp2/3 and WASp (bottom left). These podosome structures degrade ECM and basement membrane via secreted and/or transmembrane MMPs. Putative crosstalk between these protease releasing structures and proplatelet arms extending into vessels (bottom right) remains to be determined. Professional illustration by Marie Dauenheimer.
Understanding how megakaryocytes release platelets fascinates hematology researchers and has practical implications for bleeding and thrombotic diseases. Bone marrow megakaryocytes are large (≥50 μm) polyploid cells with an elaborate cytoskeletal-based demarcation system that defines cytoplasmic territories destined to form platelets.2 Maturing megakaryocytes extrude proplatelet processes that eventually fragment, releasing a shower of platelets. Italiano and colleagues first documented this dramatic process by live cell microscopy monitoring of cultured megakaryocytes.3 Junt et al4 used real-time intravital fluorescence microscopy to show that proplatelet-like structures form in the cranial marrow of live mice and gain access to the bloodstream by protruding through bone marrow vascular sinusoids. Further insight into how this occurs was provided by Sabri et al5 through studies of Wiskott Aldrich syndrome (WAS), an inherited microthrombocytopenia and immune deficiency caused by mutations in the WAS protein (WASp), a positive regulator of actin polymerization. WASp-deficient mice released platelets excessively into the bone marrow, providing a potential explanation for thrombocytopenia. The investigators showed that interaction of the bone matrix component collagen I with its receptors on normal megakaryocytes inhibits proplatelet arm formation. Moreover, on wild-type megakaryocytes, collagen I induced the formation of podosomes, “feet-like” structures composed of an actin core surrounded by scaffolding and adhesion signaling proteins.6 Podosomes form on the plasma membranes of numerous specialized cells to facilitate their motility and invasiveness, in part by elaborating ECM proteases.7 Remarkably, WASp-deficient megakaryocytes were unable to suppress proplatelet arm formation or generate podosomes normally in response to collagen I and SDF-1. This led to the model that collagen I inhibits the formation of proplatelet arms, pending the formation of podosomes that promote invasion through blood vessel walls. WASp promotes podosome formation by stimulating actin polymerization.8 In WAS, megakaryocytes lose their ability to extend proplatelet processes into the bloodstream and platelets are released abnormally into the bone marrow, causing thrombocytopenia and bleeding diathesis.
In the current study, Schachtner et al validate this model further by characterizing megakaryocyte podosome formation extensively and demonstrating their ability to degrade ECM.1 The study demonstrates that megakaryocytes cultured from murine bone marrow or human umbilical cord blood generate classical podosomes with F-actin cores surrounded by rings of the cytoskeletal protein vinculin. This process was associated with actin polymerization induced by Arp2/3, a cytoskeletal organizing complex known to be activated by WASp. Megakaryocyte podosome formation not only occurred in response to collagen I, as shown by Sabri et al, but was also stimulated by other substrates, including fibrinogen, as observed in other podosome-forming hematopoietic cells.7 Interestingly, podosome numbers, density, and lifespan were influenced by the nature of the substrate and cytokine stimuli, suggesting that the process is highly tuned and exquisitely responsive to the extracellular environment. For example, podosomes were increased by SDF-1, which also acts as a chemotaxis factor for megakaryocytes in the bone marrow niche.9 A key feature of podosomes is their proteolytic degradation of ECM, as this report demonstrates for the first time in megakaryocytes. Specifically, investigators showed that megakaryocyte podosomes open holes in an artificial matrix composed of fluorescently labeled fibrinogen. Additionally, sophisticated microscopy studies showed that green fluorescent protein–expressing megakaryocytes spread over a native basement membrane formed actin-rich foci that, over time, protruded into the membrane in a matrix metalloprotease (MMP)-dependent fashion. Megakaryocyte podosome degradation of fibrinogen and invasion into basement membrane was sensitive to general MMP inhibitors, although the particular proteases responsible were not identified. Due to technical difficulties, the investigators could not demonstrate directly that megakaryocyte podosomes degrade collagen, which may represent a key in vivo substrate.
The findings in this study suggest that podosomes play a pivotal role in megakaryocyte remodeling of ECM, transitioning from endosteal to vascular niches, and depositing platelets into the bloodstream (see figure). This work further elucidates current models for thrombopoiesis, the pathophysiology of WAS, and perhaps other thrombocytopenic disorders. The findings are also relevant for current efforts to generate platelets in vitro for transfusion therapies. One important next step will be to characterize more thoroughly podosome formation and function in human megakaryocytes. Schachtner et al showed that umbilical cord blood–derived megakaryocytes form podosomes, but their ECM degradative capacity has yet to be shown. Human megakaryocytes can be generated in vitro from numerous cell types, including induced pluripotent stem cells.10 By using induced pluripotent stem cells derived from patients with platelet diseases such as WAS and May-Hegglin anomaly, the role for the causative genes and their proteins in human megakaryocyte podosome dynamics can be analyzed. Another immediate area for exploration is to identify the specific megakaryocyte podosome proteases that degrade ECM. MMP-9 is implicated in SDF-1–mediated migration of megakaryocytes9 and it should now be possible to investigate this axis in podosome formation. It will also be interesting to investigate further how bone marrow and vascular niches differentially regulate the formation of megakaryocyte podosomes and proplatelet arms and whether these two processes are physically or mechanistically linked. Overall, the work by Schachtner et al provides novel insights and strategies for exploring mechanisms involved in megakaryocyte migration and platelet biogenesis.
Conflict-of-interest disclosure: The author declares no competing financial interests.