Key Points
CD161 defines proinflammatory FoxP3+ cells that have classic Treg signatures, yet share effector T-cell properties.
CD161+ Treg proinflammatory phenotype is stable upon Treg expansion and thus should be considered in therapeutic strategies using Treg.
Abstract
Regulatory FoxP3+CD4+ T cells (Treg) are vital for maintaining the balance between tolerance, adequate immune response, and autoimmunity. Despite this immunoregulatory role, it has been shown that Treg may also produce proinflammatory cytokines. Here we present a distinct population of Treg, defined by CD161 expression, as the major source of FoxP3+ Treg-derived proinflammatory cytokines. CD161+ Treg can be followed throughout development, from thymus and cord blood to healthy child and adult samples. CD161+ Treg display anergy, are suppressive in cocultures with conventional T cells (Tconv), and possess a predominantly demethylated Treg-specific demethylated region of the FOXP3 locus. In addition to the production of interleukin (IL) 17A, interferon γ, and IL-2, CD161+FoxP3+ cells share markers with Tconv, including expression of the transcription factors retinoic acid-related orphan receptor Cv2 (RORCv2) and T-cell-specific T-box transcription factor (Tbet). Expression of CD161 and enrichment for cytokine production are stable characteristics of CD161+ Treg upon both short- and longer-term culture in vitro. Additionally, CD161+ Treg are highly enriched within the inflammatory environment of childhood arthritis, suggesting a role in disease. Our data therefore demonstrate that CD161+FoxP3+ T cells are a novel Treg subset, found in health and disease, which display high proinflammatory potential but also exhibit hallmark Treg characteristics.
Introduction
The maintenance of immune tolerance is a key function of the immune system, mediated in part by the proper functioning of regulatory cells. One important population of regulatory CD4+ T cells (Treg) is defined by expression of the transcription factor FoxP3, which is essential for their suppressive capacity toward effector cells. Treg are thought to arise either early in ontogeny in the thymus (natural Treg) or by induction in the periphery (induced Treg). Given the position of FoxP3 as a master regulator of the Treg program, genetic mutations to the FOXP3 gene can lead to severe autoimmunity, as exemplified by the multiple immunopathologies exhibited by patients with immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome.1,2
In recent years, several different subsets of FoxP3+ Treg have been identified. Memory Treg have been proposed, defined by lack of CD45RA expression3 and/or CC chemokine receptor (CCR) 6 expression.4 CCR6 is also an important chemokine receptor found predominantly on effector T helper (Th) 17 cells. Similarly, Treg expressing the Th1-associated chemokine receptor CXC chemokine receptor 3 have been identified.5 Thus, it has been proposed that differing chemokine receptor expression patterns on Treg confers the ability of such cells to colocalize with, and therefore regulate, different types of immune response.6
Many links between Treg and Th17 cells have been established, possibly indicating their coevolutionary development.7 We have previously shown that Treg and Th17 cells have a reciprocal relationship at the site of inflammation in autoimmune childhood arthritis,8 and a higher frequency of Treg is associated with a milder disease course.9 The mere presence of Treg, however, does not always ensure tolerance, as functionality is vital. A central function of Treg is suppression of inflammatory responses, and it was previously thought that a hallmark of Treg was their lack of cytokine production.10,11 However, recent studies have demonstrated that a small proportion of Treg is able to produce proinflammatory cytokines.12,13 Following ex vivo isolation, these cytokine-producing Treg were still able to suppress in vitro and showed demethylation of the Treg-specific demethylated region (TSDR) of the FOXP3 gene, a feature that is widely considered to be an epigenetic requisite for a stable Treg program.12,14
Other studies, which took the approach of expanding or stimulating Treg in vitro, suggested that interferon γ (IFN-γ) or interleukin (IL) 17A could be induced, depending on culture conditions.15,16 These in vitro manipulated Treg were still able to suppress conventional T cells (Tconv) proliferation. These interesting observations imply that the boundaries between Tconv and Treg programs may be more blurred than previously thought and raise several important questions, in particular, how such cells arise in vivo in humans and their functional relevance in health and disease. In addition, a marker to identify such “hybrid” Treg cells would be of great interest to those who wish to employ Treg therapies.
In this paper, we have identified the lectin-like receptor, CD161, as the marker of FoxP3+ Treg in humans that harbor proinflammatory potential. CD161 expression has previously been linked to Th17 cells,17 but we believe this to be the first report that definitively links CD161 expression to the population of FoxP3+ cells that display inflammatory signatures. We report the novel findings that CD161+ Treg are bona fide suppressors and display epigenetic modifications associated with stable FoxP3 expression. The cytokine-producing phenotype of CD161+FoxP3+ cells persists over longer-term Treg expansion. CD161+ Treg exist in healthy individuals across a wide range of ages and tissues. Additionally, we show that such cells are increased at the sites of inflammation in children with autoimmune arthritis. We propose that such cells may play important roles in dictating the balance between immunity and tolerance at sites of infection and inflammation. However, given their inflammatory potential, we suggest that CD161+ Treg need to be taken into account in Treg preparation that may be used for adoptive transfer cellular therapies in human.
Materials and methods
Human samples and cells
Blood samples were obtained from 39 healthy adult volunteers and 12 healthy children (aged 1-10 years; male/female, 7:4), all with no known autoimmune or genetic conditions. Umbilical cord blood (UCB) samples were obtained from placental cords after normal delivery of healthy infants. Forty-eight patients with juvenile idiopathic arthritis (JIA; aged 3-16 years; male/female, 8:36) were included in this study, of which 37 had oligoarticular JIA (O-JIA) and 11 polyarticular JIA (P-JIA) as classified according to internationally agreed criteria.18 Synovial fluid (SF) mononuclear cells, peripheral blood mononuclear cells (PBMC), and UCB mononuclear cells were prepared by density gradient centrifugation using LymphoPrep (Axis-Shield) with SF samples undergoing pretreatment with hyaluronidase (10 U/mL; Sigma-Aldrich).8 Human thymus samples were obtained after surgical removal in children undergoing corrective cardiac surgery. Thymocytes were isolated by gentle tissue disruption followed by sieving into RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin/streptomycin (Life Technologies) and used immediately. The study had full ethical approval from the Local Research Ethics Committee; in accordance with the Declaration of Helsinki, fully informed consent was obtained from parents of all children included (with age-appropriate child assent) and from adult donors.
Flow cytometry and cell sorting
Five- to 8-color flow cytometry was performed with directly conjugated antibodies (supplemental Table 1; see the Blood Web site) using standard techniques19 ; dead cells were excluded using LIVE/DEAD Fixable blue Dead Cell Stain (Life Technologies). Intracellular staining for cytokines and transcription factors was performed using a FoxP3 buffer kit (eBioscences). Cytokine production was assessed after 3-hour stimulation of cells at 37°C in the presence of phorbol 12-myristate 13-acetate (PMA) (0.05 μg/mL), ionomycin (0.5 μg/mL), and brefeldin A (5 μg/mL) (Sigma-Aldrich).
Cell sorting was performed with a MoFlo XDP cell sorter (Beckman Coulter) after preenrichment of CD4+ T cells by immunomagnetic negative selection (Stemcell Technologies). Gates were set on single lymphocyte population; dead cells were excluded based on their uptake of 4,6 diamidino-2-phenylindole stain (Sigma-Aldrich). Treg were defined as CD4+CD25hiCD127lo cells; Tconv were defined as CD4+CD25–CD127hi cells. Purity and FoxP3 expression was assessed on all sorted cells. Unless specified, sorted subsets reaching ≥90% purity based on FoxP3+ (Treg) or FoxP3– (Tconv) staining were used in assays.
Real-time polymerase chain reaction (RT-PCR)
Messenger RNA (mRNA) was extracted from sorted cells according to the manufacturer’s instructions (Trizol; Life Technologies) and converted to complementary DNA using priming with random hexamers.19 RT-PCR was performed using SYBR-green (Biorad) and primers for FOXP3, RORCv2 (variant 2), TBX21 (Tbet), IL23R, and ACTB (β-actin) (supplemental Table 2; primer design using Primer320 ). Amplification was performed on the Rotorgene6000 (Corbett Life Science) with the following cycling conditions: 95°C for 5 minutes, 45 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds, followed by 5 minutes at 72°C. Melt curve and SYBR-green emission data were collected. Relative concentrations were calculated using a standard curve; values were normalized to amplification products of β-actin.
In vitro proliferation and suppression assays
To assess in vitro proliferation or anergy, sorted cells (CD161+ Treg [CD4+CD25hiCD127loCD161+], CD161– Treg [CD4+CD25hiCD127loCD161–], and/or Tconv [CD4+CD25–CD127hi]) were carboxyfluorescein diacetate succinimidyl ester (CFSE) labeled. Briefly, cells were labeled in 1 μM CFSE solution for 10 minutes at room temperature in the dark, followed by addition of an equal volume of FBS for 10 minutes at room temperature before multiple washes with medium. Labeled cells cultured at 5 × 104 per well in V-bottom 96-well plates (CoStar), coated with 1 μg/mL anti-CD3 (clone UCHT1; R&D systems) ± 5 μg/mL anti-CD28 (clone CD28.2; BD Biosciences) antibodies, in culture medium (RPMI 1640 supplemented with 10% FBS and 100 U/mL penicillin/streptomycin), at 37°C and 5% CO2. After 5 days, cells were restimulated as described previously. CFSE dilution, IL-17A, and IFN-γ production were assessed by flow cytometry.
Suppression assays were performed with 5 × 104 CFSE-labeled Tconv and unlabeled CD161+ or CD161– Treg at a 1:1 ratio under the same conditions as the proliferation assay. CFSE dilution, IL-17A, and IFN-γ production were assessed by flow cytometry. Cytokine protein levels in culture supernatants were assessed by multiplex immunoassay.21 Suppression of proliferation was calculated using the FlowJo %-divided function. The condition Tconv alone was set as 0% suppression.
CD161 expression stability
Sorted CD161+ Treg or CD161– Treg subpopulations were CFSE labeled and spiked at a frequency of 5% to 7% into cultures of 1 × 105 autologous PBMC in U-bottom 96-well plates (CoStar), precoated with 1 μg/mL anti-CD3 and 5 μg/mL anti-CD28, without cytokine or in the presence of IL-2, IL-6 (BD), or IL-12 (R&D; all 10 ng/mL), at 37°C in 5% CO2. After 3 days, cells were restimulated, and CD161 expression and cytokine production were assessed by flow cytometry.
For Treg expansion, 5 × 104 sorted Treg were cultured in V-bottom plates coated with 1 μg/mL anti-CD3 and 5 μg/mL anti-CD28, in the presence of 100 U/mL IL-2 (Roche) for 4, 6, or 10 days. IL-2 was renewed every 2 days. At the end of the culture, CD161 and FoxP3 expression and cytokine production were assessed by flow cytometry.
FOXP3 TSDR methylation analysis
DNA was extracted from sorted cell subsets (purity of ≥80%) using the DNeasy blood and tissue kit and bisulfite treated using the Epitect Bisulfite Kit (both Qiagen) according to the manufacturer’s instructions. A 336–base pair segment containing the TSDR of FOXP3 was amplified using published primers22 using platinum high-fidelity Taq (Life Technologies), followed by cloning of PCR products (TOPO-TA kit). Twenty to 35 clones per subset were sequenced, and from these, the percentage of clones displaying methylated cytosine guanine dinucleotide (CpG) for each site and total average were determined.
Statistical tests
Statistical analysis was performed using Prism 4 for Macintosh (GraphPad) software. Data are shown as mean ± standard error of the mean (SEM). The Mann-Whitney, Wilcoxon matched pairs test, or paired t test were applied to compare 2 groups; the 1-way analysis of variance (ANOVA) with Bonferroni’s post hoc tests was used to compare 3 or more means. Best fit of correlation was measured by the root mean square. All P values < .05 were considered statistically significant. In the figures, P values are displayed according to the following scheme: ***P < .001; **P < .01; *P < .05.
Results
CD161+FoxP3+ cells exist at different development stages
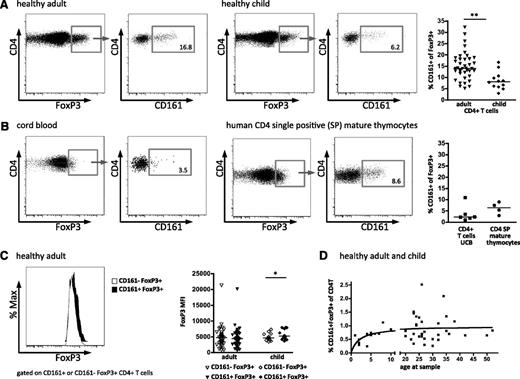
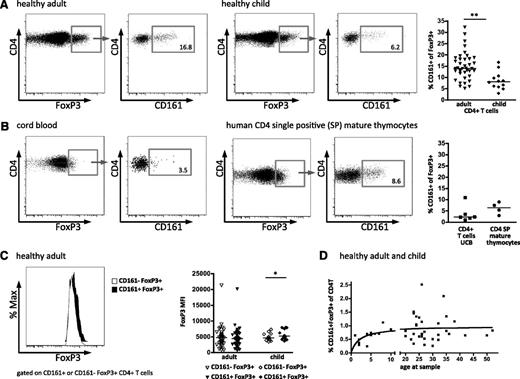
Within the CD4+ T-cell lineage, expression of CD161 has been shown to define a population of cells that contains Th17 cells, as well as “ex-Th17” cells, which have lost their initial IL-17A production and gained IFN-γ production.23,24 However, CD161 expression has not been typically associated with Treg. During our study of human Treg, we observed a subpopulation that also expresses CD161 and is present at a median frequency of 14% of FoxP3+CD4+ peripheral blood T cells in healthy adult donors (Figure 1A). Comparable results were seen with 3 different FoxP3 antibody clones, and CD161+FoxP3+ cells were CD25hiCD127lo (supplemental Figure 1). Interestingly, this population was also observed in the blood of healthy children, although at a significantly lower frequency (median, 8.1%) than in adults (Figure 1A). To assess when these CD161+FoxP3+ cells arise during development of the immune system, mature thymocytes and UCB were analyzed as sources of immunologic inexperienced and neonatal peripheral T cells. CD161+FoxP3+ cells were clearly demonstrated in both cord blood CD4+ T cells and also in mature CD4 single positive thymocytes (defined by CD1a–CD3+CD4+CD8–; Figure 1B). Together these data show that CD161+FoxP3+ cells are present within the CD4 T-cell population at different stages of development.
CD161 expression by regulatory T cells. Expression of CD161 on CD4+FoxP3+ T cells was assessed by flow cytometry. Representative fluorescence-activated cell sorter (FACS) plots of FoxP3 expression gated on CD4+ lymphocytes (left plot) and CD161 staining gated on FoxP3+CD4+ lymphocytes (right plot) in healthy adult (n = 39; left panels) and healthy child (n = 12; right panels) blood samples (summary plot far right) (A), and in cord blood (UCB; n = 5; left panels) and mature CD4 single positive thymocytes, defined by CD1a–CD3+CD4+CD8– (n = 4; right panels) (B). (C) No difference in FoxP3 protein levels between CD161–FoxP3+ and CD161+FoxP3+ Treg. Representative histograms overlay, left (white, CD161–FoxP3+; black, CD161+FoxP3+), with summary plot, right, of FoxP3 staining intensity (MFI) on CD161–FoxP3+ (clear symbols) and CD161+FoxP3+ (filled symbols) CD4+ lymphocytes for healthy adult (n = 37) and healthy child (n = 12) samples. (D) Nonlinear regression between CD161+ Treg and age in healthy child and adult samples (n = 31), 1-site–binding hyperbola. Horizontal bars represent the medians in all summary plots. Statistical analysis by Wilcoxon matched pairs test. **P < .01; *P < .05.
CD161 expression by regulatory T cells. Expression of CD161 on CD4+FoxP3+ T cells was assessed by flow cytometry. Representative fluorescence-activated cell sorter (FACS) plots of FoxP3 expression gated on CD4+ lymphocytes (left plot) and CD161 staining gated on FoxP3+CD4+ lymphocytes (right plot) in healthy adult (n = 39; left panels) and healthy child (n = 12; right panels) blood samples (summary plot far right) (A), and in cord blood (UCB; n = 5; left panels) and mature CD4 single positive thymocytes, defined by CD1a–CD3+CD4+CD8– (n = 4; right panels) (B). (C) No difference in FoxP3 protein levels between CD161–FoxP3+ and CD161+FoxP3+ Treg. Representative histograms overlay, left (white, CD161–FoxP3+; black, CD161+FoxP3+), with summary plot, right, of FoxP3 staining intensity (MFI) on CD161–FoxP3+ (clear symbols) and CD161+FoxP3+ (filled symbols) CD4+ lymphocytes for healthy adult (n = 37) and healthy child (n = 12) samples. (D) Nonlinear regression between CD161+ Treg and age in healthy child and adult samples (n = 31), 1-site–binding hyperbola. Horizontal bars represent the medians in all summary plots. Statistical analysis by Wilcoxon matched pairs test. **P < .01; *P < .05.
It has been previously shown that expression of FoxP3 protein in human T cells may be induced by cell activation and that recently activated “FoxP3 intermediate” CD4+ T cells may not have suppressive function.25 To investigate whether the CD161+FoxP3+ population has lower expression of FoxP3 compared with CD161–FoxP3+ cells, we compared the mean fluorescence intensity (MFI) of FoxP3 protein in the 2 populations. In adult donors, there was no difference in intensity of FoxP3 staining (MFI) between CD161+ and CD161–FoxP3+ cells, whereas in child donors, CD161+FoxP3+ cells actually had a slight but significantly higher median level of FoxP3 protein than CD161– cells (representative histogram and summary data; Figure 1C).
When these data were analyzed by frequency of CD161+FoxP3+ cells within the CD4+ population, across the whole age range, a rise in frequency was seen through childhood, which plateaued during the adult years; these data were best described by a 1-site–binding hyperbola (Figure 1D).
CD161+FoxP3+ cells behave like classic Treg in vitro
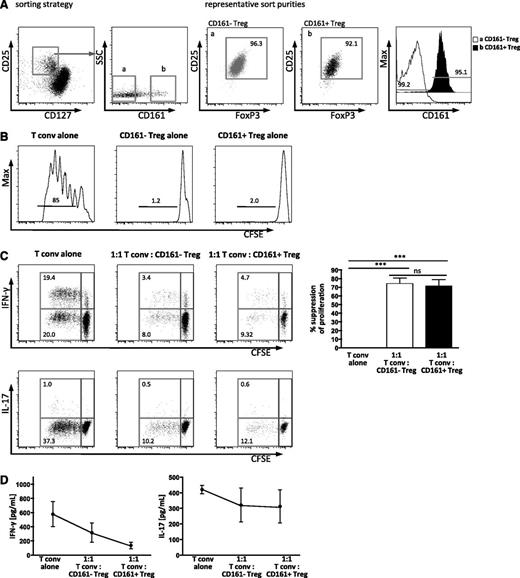
Although FoxP3 expression is one important feature of Treg, we wished to assess the functional capacity of these subpopulations. To assess the regulatory status of CD161+FoxP3+ cells, we investigated whether these cells were anergic when cultured alone in vitro, and whether they had the capacity to suppress the proliferation of conventional CD4 T cells (Tconv). Cells were sorted according to the schematic in Figure 2A to generate Tconv (CD4+CD25–CD127hi) and 2 populations of putative Treg (CD4+CD25hiCD127loCD161+ and CD4+CD25hiCD127loCD161–; sort purities of all subsets ≥90% [representative sort, Figure 2A]).
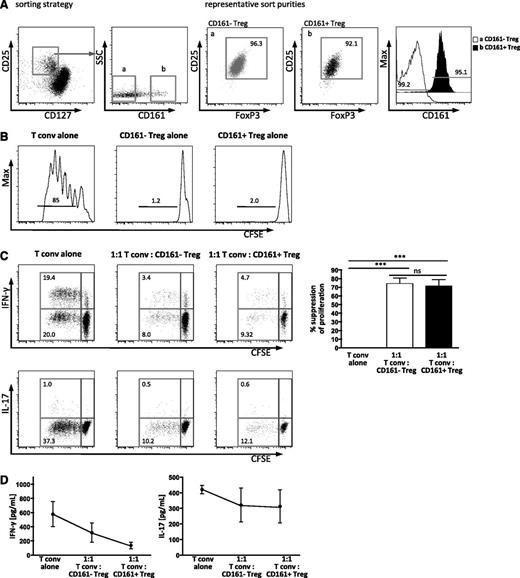
CD161+FoxP3+CD4+ T cells are suppressive and anergic in vitro. Treg were sorted by flow cytometry. (A) Sorting strategy for subpopulations of Treg defined by CD25hiCD127lo, and CD161 expression (a, CD161–; b, CD161+), with representative sort purity of ≥90% purity for both subsets for FoxP3 and CD25 (dot plots) and CD161 staining (overlay histogram). (B) Both CD161+ and CD161– Treg are anergic in vitro. Cells were sorted, labeled with CFSE, and stimulated in vitro for 5 days with anti-CD3 antibody. Representative histograms showing CFSE dilution of sorted CD161– Treg (left), CD161+ Treg (middle), and Tconv (CD25–CD127+, right) from healthy adult (1 of 2). Numbers above the bars represent the percentage of divided cells. (C) CD161+ Treg suppress proliferation and cytokine production. Sorted Tconv were labeled with CFSE, mixed with unlabeled Treg subpopulations as shown, and stimulated for 5 days as described, before analysis by flow cytometry. Representative plots show CFSE dilution, and the production of IFN-γ (upper panels) or IL-17A (lower panels) by Tconv; summary plots at day 5 for the % suppression of division of Tconv in the various cultures (n = 4; statistical analysis, 1-way ANOVA). (D) IFN-γ and IL-17A protein levels were assessed in the supernatants from cocultures by Luminex assay. Data in dot plots and histograms are gated on CD4+ lymphocytes; all summary plots show mean ± SEM. ***P < .001; ns, not significant.
CD161+FoxP3+CD4+ T cells are suppressive and anergic in vitro. Treg were sorted by flow cytometry. (A) Sorting strategy for subpopulations of Treg defined by CD25hiCD127lo, and CD161 expression (a, CD161–; b, CD161+), with representative sort purity of ≥90% purity for both subsets for FoxP3 and CD25 (dot plots) and CD161 staining (overlay histogram). (B) Both CD161+ and CD161– Treg are anergic in vitro. Cells were sorted, labeled with CFSE, and stimulated in vitro for 5 days with anti-CD3 antibody. Representative histograms showing CFSE dilution of sorted CD161– Treg (left), CD161+ Treg (middle), and Tconv (CD25–CD127+, right) from healthy adult (1 of 2). Numbers above the bars represent the percentage of divided cells. (C) CD161+ Treg suppress proliferation and cytokine production. Sorted Tconv were labeled with CFSE, mixed with unlabeled Treg subpopulations as shown, and stimulated for 5 days as described, before analysis by flow cytometry. Representative plots show CFSE dilution, and the production of IFN-γ (upper panels) or IL-17A (lower panels) by Tconv; summary plots at day 5 for the % suppression of division of Tconv in the various cultures (n = 4; statistical analysis, 1-way ANOVA). (D) IFN-γ and IL-17A protein levels were assessed in the supernatants from cocultures by Luminex assay. Data in dot plots and histograms are gated on CD4+ lymphocytes; all summary plots show mean ± SEM. ***P < .001; ns, not significant.
Both CD161+ and CD161– Treg populations were anergic (proliferation assessed by CFSE dilution) when cultured alone upon T-cell stimulation (Figure 2B). Suppression of proliferation of Tconv by Treg is considered to be a definitive property of classic Treg. The capacity of CD161– Treg and CD161+ Treg to suppress proliferation was equivalent as assessed by CFSE dilution of Tconv (Figure 2C), and upon titration of Treg, no difference was seen (data not shown). In addition, both Treg subpopulations suppressed production of IFN-γ, but not IL-17A (intracellular cytokine staining, Figure 2C; secreted cytokine in the supernatant, Figure 2D). Together these data suggest that the proposed CD161+ Treg population that we have identified, with the phenotype CD161+FoxP3+CD127loCD25hiCD4+, can be classified as regulatory in that it shows both suppressive capacity and anergy in vitro.
CD161+FoxP3+ T cells share phenotypic features with memory effector T cells
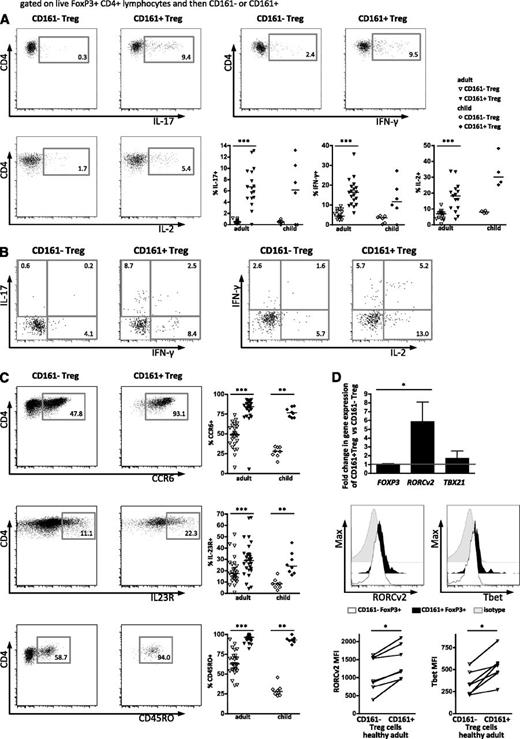
As part of their phenotype, Treg typically are not proinflammatory. However, some reports suggest that a small proportion of Treg can make proinflammatory cytokines.12,13,15 Interestingly, ex vivo cytokine staining of healthy PBMC showed a higher frequency of cells producing proinflammatory cytokines within the CD161+FoxP3+ population than the CD161–FoxP3+ population (Figure 3A). The CD161+FoxP3+ population had a significantly higher proportion of cells that produce IL-17A, IFN-γ, and surprisingly IL-2, with low or absent cytokine production within the CD161–FoxP3+ population. A proportion of CD161+FoxP3+ cells expressed more than 1 cytokine (Figure 3B). These distinct functional features were clearly apparent in blood from both adults and children, where the majority of cytokine-producing Treg is defined by CD161 expression. CD127 has been shown to inversely correlate with FoxP3 expression and suppressive functions in Treg26 : cytokine-producing CD161+FoxP3+ cells were also CD127 low (supplemental Figure 2A). Because intracellular cytokine staining may not correlate precisely with secretion, we investigated the capacity of Treg to release the proinflammatory cytokine IL-17. Following cytokine capture, intracellular FoxP3 staining was performed, and cells were analyzed based on their expression of CD161 (supplemental Figure 2B). Captured IL-17A amount was comparable with intracellular cytokine staining, showing that IL-17A is produced and secreted by FoxP3+CD161+ cells. Furthermore, CD161+ Treg produced cytokines during suppression assays (supplemental Figure 2C).
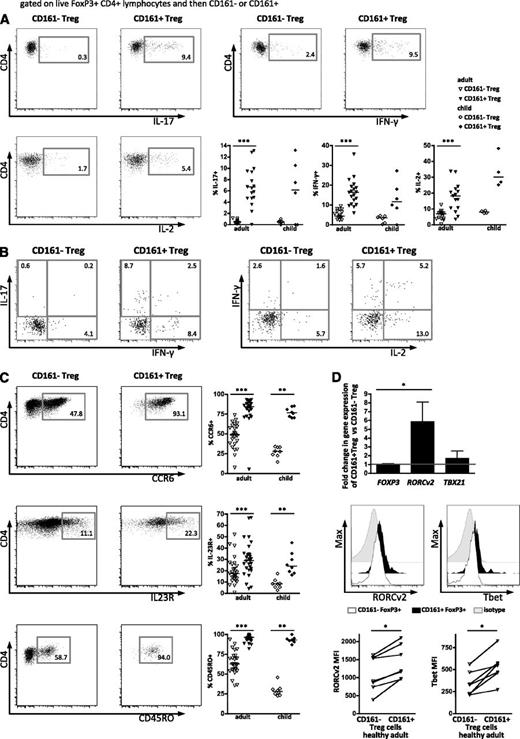
CD161+FoxP3+ regulatory T cells share phenotypic features with memory effector T cells. (A) Production of cytokines ex vivo in Treg subpopulations was assessed by intracellular flow cytometric staining after stimulation with PMA and ionomycin. Dot plots show intracellular cytokine staining for IL-17A (top left), IFN-γ (top right), and IL-2 (bottom left) gated on FoxP3+CD161– and FoxP3+CD161+ peripheral blood Treg, respectively, in 1 representative healthy adult, with summary plots (bottom right) of staining on healthy adult (n = 13-19) and child (n = 4-6) peripheral blood samples. (B) Representative staining showing double positive cytokine production (left, IL-17A vs IFN-γ; right, IFN-γ vs IL-2) comparing CD161–FoxP3+ to CD161+FoxP3+ cells. (C) Expression of effector cell markers on CD161+FoxP3+ cells. Representative plots showing expression of CCR6 (top), IL-23R (middle), and CD45RO (bottom) on FoxP3+CD161– and FoxP3+CD161+ Treg, respectively, with summary plots (right) for healthy adult (n = 16-28) and child (n = 6-8) peripheral blood samples. (D) Transcription factor expression in CD161+FoxP3+ cells. Fold change in mRNA expression of FOXP3, RORCv2, and TBX21 (Tbet) in sorted CD161+ Treg relative to sorted CD161– Treg assayed by RT-PCR (n = 3-6; top). RORCv2 and Tbet protein expression was assessed by flow cytometry ex vivo. Representative FACS plots of expression of RORCv2 (middle left) and Tbet (middle right) in CD161–FoxP3+ (open histogram) compared with CD161+FoxP3+ cells (filled histogram) and isotype (gray histogram); summary graphs of FACS data are shown below (n = 7). Data in dot plots and histograms are derived from gated CD4+FoxP3+ lymphocytes. In scatter plots, horizontal lines represent median; in bar graph, bars represent mean ± SEM with Wilcoxon matched pairs test between cell subsets, and between groups 1-way nonparametric ANOVA (Kruskal-Wallis with Dunn’s test). ***P < .001; **P < .01; *P < .05.
CD161+FoxP3+ regulatory T cells share phenotypic features with memory effector T cells. (A) Production of cytokines ex vivo in Treg subpopulations was assessed by intracellular flow cytometric staining after stimulation with PMA and ionomycin. Dot plots show intracellular cytokine staining for IL-17A (top left), IFN-γ (top right), and IL-2 (bottom left) gated on FoxP3+CD161– and FoxP3+CD161+ peripheral blood Treg, respectively, in 1 representative healthy adult, with summary plots (bottom right) of staining on healthy adult (n = 13-19) and child (n = 4-6) peripheral blood samples. (B) Representative staining showing double positive cytokine production (left, IL-17A vs IFN-γ; right, IFN-γ vs IL-2) comparing CD161–FoxP3+ to CD161+FoxP3+ cells. (C) Expression of effector cell markers on CD161+FoxP3+ cells. Representative plots showing expression of CCR6 (top), IL-23R (middle), and CD45RO (bottom) on FoxP3+CD161– and FoxP3+CD161+ Treg, respectively, with summary plots (right) for healthy adult (n = 16-28) and child (n = 6-8) peripheral blood samples. (D) Transcription factor expression in CD161+FoxP3+ cells. Fold change in mRNA expression of FOXP3, RORCv2, and TBX21 (Tbet) in sorted CD161+ Treg relative to sorted CD161– Treg assayed by RT-PCR (n = 3-6; top). RORCv2 and Tbet protein expression was assessed by flow cytometry ex vivo. Representative FACS plots of expression of RORCv2 (middle left) and Tbet (middle right) in CD161–FoxP3+ (open histogram) compared with CD161+FoxP3+ cells (filled histogram) and isotype (gray histogram); summary graphs of FACS data are shown below (n = 7). Data in dot plots and histograms are derived from gated CD4+FoxP3+ lymphocytes. In scatter plots, horizontal lines represent median; in bar graph, bars represent mean ± SEM with Wilcoxon matched pairs test between cell subsets, and between groups 1-way nonparametric ANOVA (Kruskal-Wallis with Dunn’s test). ***P < .001; **P < .01; *P < .05.
Given the evidence for developmental and phenotypic relationships between Treg and Th17 cells, surface markers known to be expressed on Th17 cells, such as CCR6, IL-23R,27 and the memory marker CD45RO,28 were investigated. In both adults and children, proportions of cells expressing CCR6 and IL-23R were significantly increased in CD161+FoxP3+ compared with CD161–FoxP3+ cells, whereas the majority of CD161+FoxP3+ cells were within the CD45RO+ population, even in the blood of young children (Figure 3C).
We next investigated whether the proinflammatory potential of CD161+FoxP3+ cells is associated with expression of transcription factors typical of Th1 or Th17 cells. RT-PCR for TBX21 (Tbet) and RORCv2 revealed a significant increase of the Th17-associated transcription factor RORCv2 (mean fold increase of 6.4, P < .05) and a trend toward increased Tbet mRNA (mean fold increase of 2.0), linked to IFN-γ production, in CD161+FoxP3+ compared with CD161–FoxP3+ cells (Figure 3D, top). There was no difference in the amount of FOXP3 mRNA between the 2 populations. Significant increases in protein levels of both RORCv2 and Tbet in CD161+FoxP3+ cells were also confirmed by flow cytometry (Figure 3D, middle and bottom by MFI). Furthermore, supporting previous evidence,29 in vitro antigen challenge (Candida albicans, hCMV) elicited cytokine-polarized responses in CD161+FoxP3+ but not CD161–FoxP3+ cells (supplemental Figure 3).
Together these data suggest that CD161+FoxP3+ cells are distinct in their proinflammatory cytokine production and show a memory phenotype with overlap with both Th17 and Th1 programs.
CD161+ Treg use a diverse TCR-Vβ repertoire
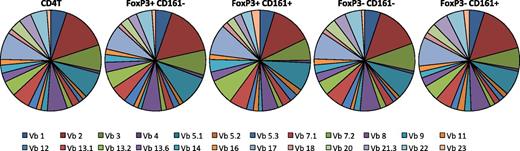
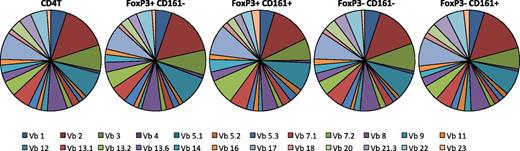
CD161 expression has been associated with invariant T-cell receptor (TCR) expression on natural killer T and some CD8+ populations, which express a limited range of TCR-Vβ chain proteins.30,31 To test the TCR-Vβ repertoire on CD161+FoxP3+ cells, flow cytometry staining for TCR-Vβ families was performed. The expression of 24 TCR-Vβ families was investigated within 5 different T-cell subsets: total CD4+, CD161–FoxP3+CD4+, CD161+FoxP3+CD4+, CD161–FoxP3–CD4+, and CD161+FoxP3–CD4+ lymphocytes (Figure 4). TCR-Vβ families examined by the panel of available antibodies covered a mean of 70.18% (± standard deviation of 8.268) of all analyzed cells, representing the majority of cells within each subset investigated. The TCR-Vβ repertoire was comparable between all subsets tested, indicating that CD161+FoxP3+ cells do not express an invariant TCR but have a diverse repertoire comparable with CD161–FoxP3+ and Tconv.
CD161+ regulatory T cells do not express an invariant TCR. Frequency of TCR-Vβ expression was assessed using a flow cytometry panel to detect 24 Vβ families, in healthy adult CD4+ T cells, CD161–FoxP3+CD4+, CD161+FoxP3+CD4+, CD161−FoxP3–CD4+, and CD161+FoxP3–CD4+ cell populations as shown (n = 5). Data were gated on the specific population and then each TCR-Vβ. Pie charts show proportion of all cells positive for a specific TCR-Vβ (which identify a mean of 70.18% of all cells within respective subset).
CD161+ regulatory T cells do not express an invariant TCR. Frequency of TCR-Vβ expression was assessed using a flow cytometry panel to detect 24 Vβ families, in healthy adult CD4+ T cells, CD161–FoxP3+CD4+, CD161+FoxP3+CD4+, CD161−FoxP3–CD4+, and CD161+FoxP3–CD4+ cell populations as shown (n = 5). Data were gated on the specific population and then each TCR-Vβ. Pie charts show proportion of all cells positive for a specific TCR-Vβ (which identify a mean of 70.18% of all cells within respective subset).
To investigate the relationship between CD161+ and CD161– Treg, the clonality of TCR was tested by sequence analysis across the variable diversity joining junction.32 CD161+ and CD161– Treg were sorted, and CDR3 regions of TCR-Vβ2 cloned and sequenced (supplemental Figure 4). Both Treg subsets showed clonality, a total of 345 sequences (170 CD161– Treg, 175 CD161+ Treg), with 122 clonal sequences for CD161– and 118 clonal sequences for CD161+ Treg, which were predominantly nonoverlapping (2 sequences found in both subsets). These data raise the possibility that CD161+ Treg may have a distinct origin from CD161– Treg.
CD161+ Treg have a predominantly demethylated TSDR of FOXP3, and their phenotype is stable in vitro
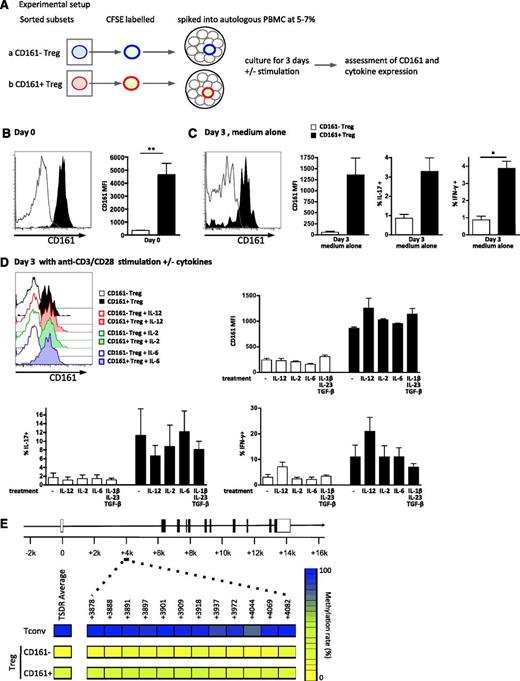
To investigate the stability of CD161 expression on Treg, CD161– and CD161+ Treg were sorted, labeled, and followed in an in vitro culture system where they were present at a frequency of 5% to 7% of unlabeled total PBMC (for schematic, see Figure 5A). Unstimulated (Figure 5C) and anti-CD3/CD28 stimulated with or without cytokine treatment conditions (Figure 5D) were tested in both systems. No differences in viability between conditions were seen (data not shown). CD161+ Treg (filled histogram and bars) retained expression of CD161 (Figure 5C-D), whereas CD161– cells (clear histograms and white bars) did not acquire CD161 expression (supplemental Figure 5). CD161 expression was stable under all conditions, although CD161 expression per cell (MFI) decreased slightly at 3 days (Figure 5C-D).
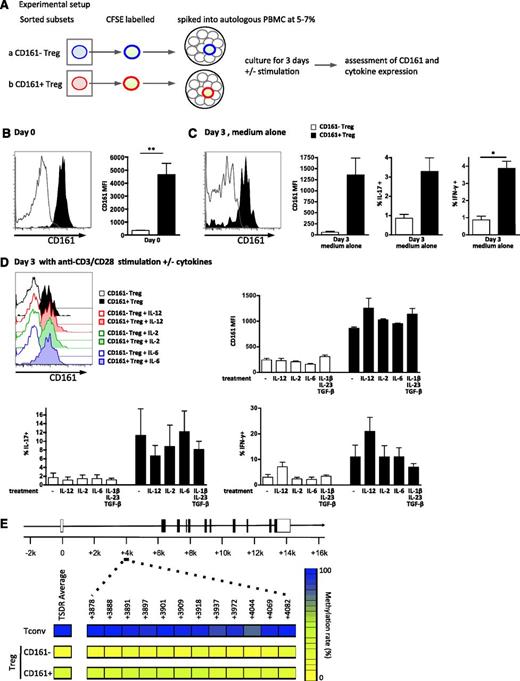
CD161+ regulatory T cells are stable in vitro and possess a predominantly demethylated TSDR. (A-D) Sorted Treg subpopulations were labeled with CFSE, “spiked” back into autologous PBMC at a frequency of 5% to 7%, and cultured for 3 days on plates coated with or without anti-CD3/CD28 and stimulated with either medium alone or cytokines as shown. Schematic of experimental setup for in vitro stability analysis is shown in panel A; representative overlay histograms of CD161 expression gated on CFSE+ cells and summary plots of CD161 MFI at day 0 (n = 5) (B), after 3-day culture in medium alone (n = 3) (C), and after 3-day culture on anti-CD3/CD28–coated plates ± cytokines (n = 3) (D, top); cytokine production by the labeled CD161– or CD161+ Treg was also assessed on day 3 (C; D, bottom). Data in histograms are events gated on CD4+CFSE+ live cells. For both histograms and graphs, clear symbols represent CD161– Treg, and filled symbols CD161+Treg. In bar graph, bars represent means ± SEM with Wilcoxon matched pairs test between cell subsets, and between groups 1-way nonparametric ANOVA (Kruskal-Wallis with Dunn’s test). **P < .01; *P < .05. (E) Both CD161– Treg and CD161+ Treg cells contain a predominantly demethylated FOXP3 TSDR. DNA was extracted from sorted Tconv, CD161– Treg, and CD161+ Treg (purity >80%) and bisulfite treated, and the TSDR was amplified and then cloned. Twenty to 35 individual clones were sequenced, and the percentage of methylated CpG islands determined. Panel E shows a schematic of the FOXP3 locus representing the location of the 12 CpG islands analyzed (positions +3878 to +4082), with mean % methylation of each CpG island displayed (left) and the average for all islands (right; blue, 100%, and yellow, 0%; 1 of 3 representative individuals shown).
CD161+ regulatory T cells are stable in vitro and possess a predominantly demethylated TSDR. (A-D) Sorted Treg subpopulations were labeled with CFSE, “spiked” back into autologous PBMC at a frequency of 5% to 7%, and cultured for 3 days on plates coated with or without anti-CD3/CD28 and stimulated with either medium alone or cytokines as shown. Schematic of experimental setup for in vitro stability analysis is shown in panel A; representative overlay histograms of CD161 expression gated on CFSE+ cells and summary plots of CD161 MFI at day 0 (n = 5) (B), after 3-day culture in medium alone (n = 3) (C), and after 3-day culture on anti-CD3/CD28–coated plates ± cytokines (n = 3) (D, top); cytokine production by the labeled CD161– or CD161+ Treg was also assessed on day 3 (C; D, bottom). Data in histograms are events gated on CD4+CFSE+ live cells. For both histograms and graphs, clear symbols represent CD161– Treg, and filled symbols CD161+Treg. In bar graph, bars represent means ± SEM with Wilcoxon matched pairs test between cell subsets, and between groups 1-way nonparametric ANOVA (Kruskal-Wallis with Dunn’s test). **P < .01; *P < .05. (E) Both CD161– Treg and CD161+ Treg cells contain a predominantly demethylated FOXP3 TSDR. DNA was extracted from sorted Tconv, CD161– Treg, and CD161+ Treg (purity >80%) and bisulfite treated, and the TSDR was amplified and then cloned. Twenty to 35 individual clones were sequenced, and the percentage of methylated CpG islands determined. Panel E shows a schematic of the FOXP3 locus representing the location of the 12 CpG islands analyzed (positions +3878 to +4082), with mean % methylation of each CpG island displayed (left) and the average for all islands (right; blue, 100%, and yellow, 0%; 1 of 3 representative individuals shown).
It has been suggested that both IL-12 and IL-2 have an effect on CD161 expression.33,34 IL-12 is linked to IFN-γ production by T cells35 ; IL-2 is an important survival factor for Treg36,37 ; and IL-6, transforming growth factor β (TGF-β)+IL-1β+IL-23 can drive IL-17A production.38,39 IL-6 and IL-12 are also abundant at inflammatory sites, for example, within affected joints of children with arthritis.23,40 Therefore, we tested if we could further “skew” the phenotype of CD161+ Treg by culturing in the presence of cytokines. Addition of IL-12, IL-6, IL-2, or TGF-β+IL-1β+IL-23 to the culture did not alter stability of CD161 expression, although a trend toward increased CD161 levels was demonstrated when IL-12 was present (Figure 5D, top). Additionally, CD161+FoxP3+ cells maintained their cytokine phenotype, producing IL-17A and IFN-γ without further stimulation (Figure 5C, right and far right) and after T-cell activation (Figure 5D, bottom). Addition of cytokines to the culture system showed that as expected IL-12 enhanced IFN-γ production and limited IL-17A production, whereas IL-2, IL-6, and TGF-β+IL-1β+IL-23 had little effect (Figure 5D, bottom).
Recently, the importance of epigenetic modifications for Treg stability has emerged.14,22 DNA demethylation at the TSDR of the FOXP3 locus has been deemed crucial for Treg stability. CD161– Treg and CD161+ Treg both showed a predominantly demethylated TSDR (Figure 5E). Twelve CpG islands within the TSDR of the FOXP3 locus were analyzed for sorted Tconv, CD161– Treg, and CD161+ Treg (top to bottom, purity >80%). Across 3 male individuals, the TSDR of Tconv showed full methylation (96.4% [±0.8]), CD161– Treg showed a small residual methylation (7.4% [±2.5]), and CD161+ Treg showed 28.5% (±2.1).
Together these data suggest that CD161+ Treg are a stable subset with predominantly demethylated TSDR at the FOXP3 locus and that they retain CD161 expression upon TCR stimulation, whereas CD161– Treg did not gain CD161 expression.
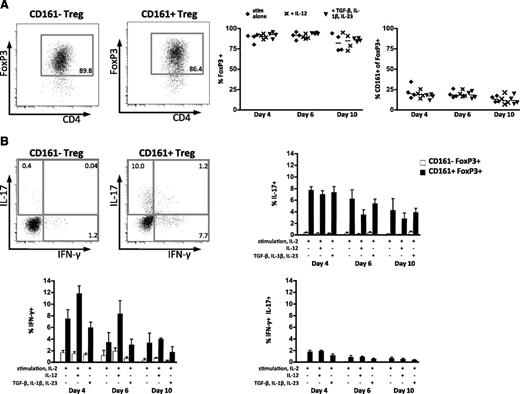
The ex vivo characteristics of CD161+ Treg are maintained during longer-term in vitro culture
To test CD161+ Treg characteristics in longer-term culture, Treg were sorted to high purity and cultured under Treg expansion conditions (plate-bound anti-CD3, anti-CD28, and IL-2 every 2 days) with or without cytokines (IL-12 for Th1-like skewing; TGF-β+IL-1β+IL-23 as IL-17A–skewing conditions). There was no difference in FoxP3 stability between CD161– and CD161+ Treg, and the frequency of CD161+FoxP3+ cells was constant throughout the 10-day culture, irrespective of conditions (Figure 6A). Furthermore, CD161+FoxP3+ cells maintained their capacity to produce IL-17A and IFN-γ, whereas cytokine production remained negligible in CD161–FoxP3+ cells. Cytokines added had little effect, with a small increase in IFN-γ and slight decrease in IL-17A in the presence of IL-12 (Figure 6B). This demonstration of persistence of CD161+FoxP3+ Treg proinflammatory potential should be of interest to investigators attempting to expand Treg ex vivo for potential therapeutic applications.
CD161+ regulatory T cells and cytokine-producing phenotype persist upon Treg expansion. Treg expansion over 10 days with or without cytokine treatment (n = 4). (A) Representative flow cytometry plots of FoxP3 expression at day 10 with stimulation alone for CD161– and CD161+ cells (left), summary plots for FoxP3 expression overall (middle), and frequency of CD161 in FoxP3+ cells (right). Horizontal lines represent the median; diamonds, stimulation alone; crosses, +IL-12; and triangles, +TGF-β, IL-1β, and IL-23. (B) Representative flow cytometry plots showing cytokine production (IL-17A vs IFN-γ) of CD161–FoxP3+ (left) and CD161+FoxP3+ cells (right) with summary plots showing mean ± SEM for IL-17A single-, IFN-γ single-, and IL-17A+IFN-γ double-producing cells for CD161– (white bars) and CD161+FoxP3+ (black bars).
CD161+ regulatory T cells and cytokine-producing phenotype persist upon Treg expansion. Treg expansion over 10 days with or without cytokine treatment (n = 4). (A) Representative flow cytometry plots of FoxP3 expression at day 10 with stimulation alone for CD161– and CD161+ cells (left), summary plots for FoxP3 expression overall (middle), and frequency of CD161 in FoxP3+ cells (right). Horizontal lines represent the median; diamonds, stimulation alone; crosses, +IL-12; and triangles, +TGF-β, IL-1β, and IL-23. (B) Representative flow cytometry plots showing cytokine production (IL-17A vs IFN-γ) of CD161–FoxP3+ (left) and CD161+FoxP3+ cells (right) with summary plots showing mean ± SEM for IL-17A single-, IFN-γ single-, and IL-17A+IFN-γ double-producing cells for CD161– (white bars) and CD161+FoxP3+ (black bars).
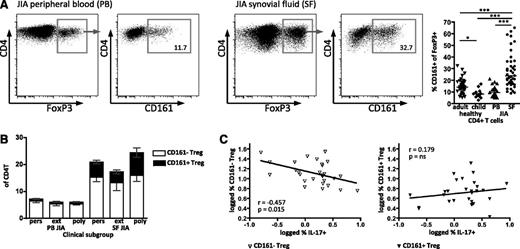
CD161+ Treg are enriched in the inflammatory environment
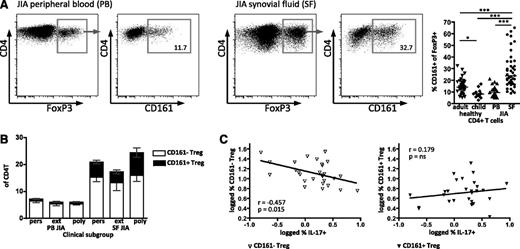
We have previously shown that Treg are greatly enriched within the highly inflammatory environment of the synovial space in JIA. We hypothesized that Treg expressing CD161 may be present within the synovial Treg population. We therefore investigated CD161 expression on Treg in O-JIA (persistent oligoarticular [affected joint count stable <5] and extended oligoarticular [affected joint count increased to ≥5 after 6 months]) and the more severe clinical type P-JIA (at onset, ≥5 joints involved): these JIA subtypes show localized inflammation restricted to the joints without systemic or major organ involvement.18 PBMC of JIA patients showed no difference to the age-matched healthy control in CD161 frequency of FoxP3+ (Figure 7A, right). However, cells from the SF of affected joints (SF mononuclear cells) had a significantly higher CD161+ frequency within the FoxP3+ population (median, 23.7%) compared with JIA PBMC (median, 10.8%) and healthy samples (median adult, 14.0%; child, 8.1%; Figure 7A), concurring with our previous findings in JIA, of skewed CD4+ populations at the inflamed site.19,41 Synovial CD161+FoxP3+ cells had the same phenotype and were potent producers of proinflammatory cytokines as the healthy control CD161+ Treg (Figure 3; JIA data not shown).
CD161 expression by regulatory T cells is enriched in the inflammatory environment. (A) CD161 status of Treg was analyzed on T cells from peripheral blood (PB) and joint (SF) of children with JIA. Representative FACS plots of FoxP3 expression on CD4+ lymphocytes and CD161 staining on FoxP3+CD4+ lymphocytes from JIA PB and JIA SF; summary plot (right) shows data for healthy adult PB (n = 39), healthy child PB (n = 12), JIA PB (n = 24), and JIA SF (n = 41) samples. Data in dot plots are gated on CD4+ lymphocytes (left plots) and then (right plots) on FoxP3+ T cells; horizontal lines represent median with Kruskal-Wallis test with Dunn’s multiple comparison test. ***P < .001; **P < .01; *P < .05; ns, not significant. (B) Frequency of CD161+ Treg in different clinical JIA phenotypes was analyzed by flow cytometry. Frequency of CD161– Treg (clear) and CD161+ Treg (black) within CD4+ T cells; data are divided into clinical subgroups, persistent O-JIA (pers), extended O-JIA (ext), and P-JIA (poly) for PB (n = 8, n = 8, and n = 5, respectively) and SF (n = 15, n = 11, and n = 11, respectively) samples. Bars represent mean ± SEM. (C) The inverse relationship between Th17 and Treg is specific to the CD161– Treg population. Correlation between SF CD161– Treg (left, clear symbols) and CD161+ Treg (right, filled symbols) with IL-17A+ CD4+ T cells within the same sample (n = 28).
CD161 expression by regulatory T cells is enriched in the inflammatory environment. (A) CD161 status of Treg was analyzed on T cells from peripheral blood (PB) and joint (SF) of children with JIA. Representative FACS plots of FoxP3 expression on CD4+ lymphocytes and CD161 staining on FoxP3+CD4+ lymphocytes from JIA PB and JIA SF; summary plot (right) shows data for healthy adult PB (n = 39), healthy child PB (n = 12), JIA PB (n = 24), and JIA SF (n = 41) samples. Data in dot plots are gated on CD4+ lymphocytes (left plots) and then (right plots) on FoxP3+ T cells; horizontal lines represent median with Kruskal-Wallis test with Dunn’s multiple comparison test. ***P < .001; **P < .01; *P < .05; ns, not significant. (B) Frequency of CD161+ Treg in different clinical JIA phenotypes was analyzed by flow cytometry. Frequency of CD161– Treg (clear) and CD161+ Treg (black) within CD4+ T cells; data are divided into clinical subgroups, persistent O-JIA (pers), extended O-JIA (ext), and P-JIA (poly) for PB (n = 8, n = 8, and n = 5, respectively) and SF (n = 15, n = 11, and n = 11, respectively) samples. Bars represent mean ± SEM. (C) The inverse relationship between Th17 and Treg is specific to the CD161– Treg population. Correlation between SF CD161– Treg (left, clear symbols) and CD161+ Treg (right, filled symbols) with IL-17A+ CD4+ T cells within the same sample (n = 28).
In children with an extended O-JIA disease course, there are fewer FoxP3+ cells than in those with the mild self-remitting persistent O-JIA.8,9 When split into clinical subgroups, data in our study replicated our previous findings for both CD161–FoxP3+ and CD161+FoxP3+ cells (Figure 7B). Additionally, the fold increase of the CD161+ Treg frequency (persistent O-JIA, 7.6; extended O-JIA, 5.4; and P-JIA, 11.0) is much greater than the change in CD161– Treg (2.6, 2.5, and 3.0, respectively). In our previous study, we also showed a reciprocal relationship between the frequency of FoxP3+ and IL-17A–producing CD4 lymphocytes in the inflammatory joint compartment.8 Analysis of our data in this study for CD161– and CD161+FoxP3+ populations demonstrated that only the CD161–FoxP3+ cells show this reciprocal relationship (r = −0.457; P = .015) but not CD161+FoxP3+ (r = 0.179; P = not significant) (Figure 7C). Together, these data demonstrate that CD161+FoxP3+ Treg are highly enriched within an inflammatory environment.
Discussion
It is widely considered that an important function of Treg is the suppression of disproportionate immune responses. Thus, Treg are an appealing target to use therapeutically. However, there are still many unknown elements to Treg therapy. Of topical relevance is the controversy surrounding the proinflammatory potential of Treg, which is present in expanded Treg populations, particularly in inflammatory settings.
In this report, we have demonstrated the existence of a distinct population of FoxP3+CD4+ Treg, defined by the expression of the Th17-associated marker CD161, which shows proinflammatory potential, but also characteristic signatures and behavioral properties of classic Treg. This population can be followed throughout life; CD161+FoxP3+ cells can be found already within the immunologic inexperienced lymphocyte population of UCB and emerge as mature single positive thymocytes along with natural Treg.
CD161+ Treg and CD161– Treg are diverse in their TCR-Vβ family repertoire. Importantly, CD161+ Treg fulfill the hallmark features of classic Treg as they suppress the proliferation of Tconv to the same extent as CD161– Treg and show a predominantly demethylated TSDR of the FOXP3 locus. Overall methylation levels were in keeping with those for previously published Treg populations3,22,42 ; although in 3 individuals tested, CD161+ Treg displayed a less demethylated TSDR overall compared with CD161– Treg. The functional consequences of this potentially intermediate demethylation state warrant future investigation.
We therefore report, to our knowledge for the first time, that CD161 expression defines the major source of cytokine-producing FoxP3+ T cells. We report that CD161+ Treg share some characteristics with Tconv phenotypes, with shared cytokine and chemokine receptors, memory markers, as well as the presence of Tconv-associated transcription factors RORCv2 and Tbet. Therefore, CD161 defines a population of “hybrid Treg” that has both inflammatory and suppressive potentials. Furthermore, we have established that cytokine-producing CD161+FoxP3+ cells are stable on expansion in vitro, whereas CD161–FoxP3+ cells do not upregulate cytokine production, even in Th1- or Th17-skewing conditions. The precise role in vivo of cytokine-producing CD161+FoxP3+ cells is worthy of further characterization.
Our data are supported by recent findings of cytokine-producing Treg that were generated by in vitro stimulation, expansion, or cloning, which were still suppressive.12,13,15,16 However, in those studies, no potential origin or definitive way of identifying these cells without ex vivo stimulation or permeabilization was given. Here we suggest that CD161 defines the major source of Treg with inflammatory potential. CD161-expressing Tconv are the precursors for Th17, and Th1 cells that have converted from Th17 cells are exclusively within the CD161+ population.17,23,24 Our data again support a link between CD161 and IL-17 production, which appears to be the case in Treg as well.
This growing recognition that Treg may have proinflammatory potential has significant implications for therapeutic adoptive transfer of human Treg because standard sorting methods to purify Treg would include such cells. Our data suggest that the inclusion of a depletion step, to remove CD161+ Treg, could reduce dangers of such proinflammatory cells in certain conditions. However, so-called specialized Treg have been shown to have the ability to regulate specific Th cell responses in mouse models and thus may have potential benefit in some conditions.5
Since their discovery, Treg have been subdivided into various groups. One functionally distinct Treg subset described was based on HLA-DR protein expression.11 Different mechanisms of suppression were shown, and the authors demonstrated the importance of both subsets, with DR+ Treg initiating early contact-dependent and DR– late FoxP3-associated suppression. Later, it was identified that IL-17–secreting Treg lie within the DR– population.15 However, in healthy individuals, most Treg are DR– (mean of 83%; A.M.P., unpublished observations). Here we have demonstrated that CD161 expression accounts almost exclusively for IL-17A–producing Treg. Another basis for defining Treg subsets is by memory markers.3,4 CD161+ Treg fall within the CD45RO+CD45RA– population described by Miyara et al3 and the CCR6+ population described by Kleinewietfeld et al4 and therefore can be considered memory Treg.
CD161 expression on Tconv or CD8+ T cells has been associated with autoimmunity and chronic infection in previous reports. CD161 is enriched on T cells within a multitude of autoimmune conditions.17,24,43,44 Furthermore, we have now shown the enrichment of CD161+ Treg within the inflammatory joint of autoimmune childhood arthritis, implicating them in disease. In contrast, studies in HIV and AIDS have shown decreased frequency and levels of CD161 on T cells.45,46 Treg expressing CD161 have not been considered in these studies as a potentially important contributor and may warrant future attention.
The finding that CD161+ Treg are enriched at sites of inflammation is interesting. The persistence and overrepresentation of CD161+ Treg compared with CD161– Treg might be detrimental for the regulation of autoimmune responses. We propose that upon an acute insult, CD161+FoxP3+ cells may have a role in dictating the balance between tolerance and immunity, with the ability to produce proinflammatory cytokines and also to suppress an overly active immune response. CD161+ Treg may be recruited via CD161 itself, as well as through CCR6, to inflammatory areas.6,47 Additionally, the CD161 ligand lectin-like transcript 1 can be induced upon activation of many cell types, possibly in response to inflammation, which may equip such cells for activation of CD161-expressing T cells.48
It remains unclear which signals direct the nature of the cytokines produced by CD161+FoxP3+ cells, but it is likely that the cytokine milieu is central in determining their cytokine-production profile, as demonstrated for some Treg.12,15 Similarly, it remains to be seen what the direct effects of CD161 ligation are on Treg and whether this is involved in the manifestation of the proinflammatory profile. To address this in the in vitro setting is technically challenging because the stimulatory antibody against CD161 is sterically hindered by the antibodies used for sorting (A.M.P., unpublished observations), which means functional analysis on purified populations with present reagents is very limited. Because CD161+ Treg are a minor population, stimulation on bulk Treg cultures does not constitute a sensitive enough assay to determine the functionality of CD161 (A.M.P., unpublished observations), and more robust in vitro modeling systems will need to be established to address this area.
An in vivo system to test CD161+ Treg functionality would be valuable. Unfortunately, this is not readily available because human CD161 does not have an exact equivalent in mice. Although CD161 is the only natural killer receptor protein 1 family member encoded in humans, in mice there are several different and functionally diverse natural killer receptor protein 1 receptors, with different expression profiles and a maximum of 45% homology compared with human CD161 (reviewed in Yokoyama et al49 ). It therefore remains to be determined whether similar or related markers of Treg with proinflammatory potential exist, if at all, in other species.
In conclusion, we report the novel discovery that CD161 defines the major source of inflammatory cytokines by Treg. CD161+FoxP3+ T cells can be found throughout development and show an effector-like phenotype. Despite a low frequency in healthy environments, such cells are highly enriched at the site of inflammation in autoimmune JIA. CD161+ Treg may therefore be pivotal in dictating the balance between tolerance, appropriate immune response, and autoimmunity. Because the field currently lacks a clear understanding of the functions of CD161, especially on T cells, further work into the biology of CD161-expressing Treg is necessary; additionally, given the knowledge that CD161+FoxP3+ cells display persistent proinflammatory characteristics, we suggest that the CD161+ population should be taken into account in Treg isolation protocols to be used for adoptive transfer therapies.
Part of the work presented in abstract form at the annual congress of the British Society of Immunology 2011, Liverpool, United Kingdom, December 8, 2011.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank volunteers, patients, and their parents for contribution of samples, as well as hospital staff who made this study possible. The authors also thank A. Furmanski, T. Crompton, and G. Davies for contributing thymus samples; and A. Eddaoudi and flow cytometry facility staff for cell sorting. Group members are thanked for their help in sample preparation and advice.
This work was supported by Nuffield Oliver Bird Programme (A.M.P.), Arthritis Research UK (Foundation Fellow 19761 [D.B.], Career Progression fellowship 19392 [K.N.], and Vacation Scholarship [Q.W.]), and SPARKS UK (08ICH09) (S.U.).
Authorship
Contribution: All authors approved the final version to be published and had input in revising it for intellectual content and style; A.M.P. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; A.M.P., D.B., K.N., and L.R.W. were responsible for the experimental design; A.M.P., D.B., Q.W., and S.U. carried out the acquisition of data; and A.M.P., D.B., and L.R.W. performed analysis and interpretation of the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne M. Pesenacker, UCL Institute of Child Health, 30 Guilford Street, London, WC1N 1EH; e-mail: a.pesenacker@ucl.ac.uk.